Dynamic hydrogel mechanics in organoid engineering: From matrix design to translational paradigms
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The extracellular matrix (ECM) serves as a dynamic biomechanical regulator of cellular behavior, yet conventional 3D culture systems, such as Matrigel, lack the spatiotemporal control required to dissect mechanotransductive mechanisms in organoids. This review systematically explores the synthesis of mechanically tunable hydrogels—spanning stiffness and viscoelasticity—and their transformative applications in organoid research. By integrating natural, synthetic, and hybrid polymers, these hydrogels enable precise recapitulation of tissue-specific ECM mechanics, overcoming limitations of batch variability and static properties. We categorize hydrogel design strategies, emphasizing crosslinking paradigms (physical vs. chemical) and dynamic bond engineering, which permit real-time modulation of mechanical cues. Applications across developmental organoids (intestinal, hepatic, renal, neural) reveal stiffness-dependent morphogenesis, where optimal mechanical niches enhance maturation via YAP/Notch signaling. Tumor organoid models (breast, pancreatic, colorectal) further demonstrate how matrix stiffening drives malignancy through mechanosensitive pathways, such as epithelial-mesenchymal transition and drug resistance. Emerging viscoelastic hydrogels, tailored via alginate molecular weight or decellularized ECM, replicate dynamic tissue mechanics, advancing cartilage and cerebellar organoid models. Critically, this review highlights innovations in programmable hydrogels that bridge 2D reductionist models and in vivo complexity, offering unprecedented insights into ECM-driven organogenesis and disease progression. Future directions include integrating bioprinting and organ-on-a-chip technologies to achieve vascularized, patient-specific organoids. By synthesizing design principles and mechanobiological mechanisms, this work establishes a roadmap for next-generation biomaterials, accelerating translational applications in drug screening, regenerative medicine, and personalized oncology.
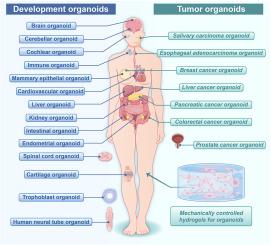
类器官工程中的动态水凝胶力学:从矩阵设计到转化范例
细胞外基质(ECM)作为细胞行为的动态生物力学调节器,然而传统的3D培养系统,如Matrigel,缺乏解剖类器官机械转导机制所需的时空控制。这篇综述系统地探讨了机械可调水凝胶的合成-跨越刚度和粘弹性-及其在类器官研究中的变革性应用。通过整合天然、合成和杂化聚合物,这些水凝胶能够精确再现组织特异性ECM力学,克服了批次可变性和静态特性的限制。我们对水凝胶设计策略进行了分类,强调交联范式(物理与化学)和动态键工程,它们允许实时调制机械线索。在发育中的类器官(肠、肝、肾、神经)中的应用揭示了刚性依赖的形态发生,其中最佳的机械壁龛通过YAP/Notch信号通路促进成熟。肿瘤类器官模型(乳腺、胰腺、结肠)进一步证明了基质硬化如何通过机械敏感途径驱动恶性肿瘤,如上皮-间质转化和耐药性。新兴的粘弹性水凝胶,通过海藻酸盐分子量或脱细胞ECM定制,复制动态组织力学,推进软骨和小脑类器官模型。重要的是,这篇综述强调了可编程水凝胶的创新,它将2D还原模型和体内复杂性联系起来,为ecm驱动的器官发生和疾病进展提供了前所未有的见解。未来的方向包括整合生物打印和器官芯片技术,以实现血管化的、患者特异性的类器官。通过综合设计原理和机械生物学机制,本工作建立了下一代生物材料的路线图,加速了在药物筛选、再生医学和个性化肿瘤学方面的转化应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


