Smart responsive injectable hydrogel loaded with icariin inhibits ferroptosis and promotes nucleus pulposus repair in intervertebral disc degeneration
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-09-17
DOI:10.1016/j.bioadv.2025.214513
引用次数: 0
Abstract
Intervertebral disc degeneration (IVDD) is a leading cause of lower back pain, characterized by the ferroptosis of nucleus pulposus cells (NPCs) and imbalance in reactive oxygen species (ROS) metabolism. Icariin (ICA), an active compound from traditional Chinese medicine, scavenges ROS and inhibits ferroptosis, but its poor water solubility and low bioavailability limit clinical use. A dual-crosslinked hydrogel (HP@ICA) is developed to address these challenges. Cyclodextrin is integrated into polyvinyl alcohol to enhance ICA loading via hydrophobic interactions. Methacrylic acid and phenylboronic acid are incorporated into hyaluronic acid, enabling dynamic bonding with polyvinyl alcohol. Ultraviolet crosslinking creates a secondary crosslinked hydrogel with pH and ROS responsiveness, self-healing properties, and improved ICA solubility, increasing its water solubility tenfold. Enhanced solubility boosts ICA's free radical scavenging capacity by 103 %, suppressing ferroptosis and promoting NP cell regeneration, as demonstrated by reduced iron ion concentrations, lower malondialdehyde levels, and restored glutathione peroxidase 4 activity. Ferroptosis inhibition facilitates type II collagen production in NP cells. Mechanistic studies reveal activation of the Nrf2-GPX4 pathway as key to regeneration. These findings suggest a promising regenerative therapeutic approach for IVDD.
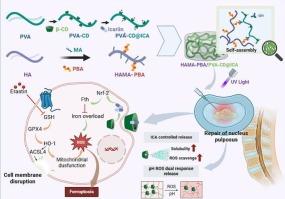
装载淫羊藿苷的灵敏可注射水凝胶抑制铁下垂并促进椎间盘退变中的髓核修复
椎间盘退变(IVDD)是腰痛的主要原因,其特征是髓核细胞(NPCs)下垂和活性氧(ROS)代谢失衡。淫羊藿苷(Icariin, ICA)是一种具有清除活性氧和抑制铁下沉作用的中药活性化合物,但其水溶性差和生物利用度低限制了其临床应用。开发了一种双交联水凝胶(HP@ICA)来解决这些挑战。环糊精被整合到聚乙烯醇中,通过疏水相互作用增强ICA负载。透明质酸中加入甲基丙烯酸和苯硼酸,使其与聚乙烯醇动态结合。紫外线交联产生了二级交联水凝胶,具有pH和ROS响应性,自修复性能,并改善了ICA的溶解度,将其水溶性提高了十倍。通过降低铁离子浓度、降低丙二醛水平和恢复谷胱甘肽过氧化物酶4活性,ICA的溶解度提高了103%的自由基清除能力,抑制铁下垂并促进NP细胞再生。抑制铁下垂促进了NP细胞中II型胶原蛋白的产生。机制研究表明Nrf2-GPX4通路的激活是再生的关键。这些发现为IVDD提供了一种有希望的再生治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


