pH-responsive 2D niobium carbide nanosheets for targeted circPUM1 siRNA delivery in ovarian cancer therapy
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Ovarian cancer progression is closely associated with tumor microenvironment (TME) dysregulation, particularly pathological angiogenesis driven by exosome-mediated crosstalk. Here, we elucidate that ovarian cancer-derived circPUM1 promotes angiogenesis by transferring to vascular endothelial cells via exosomes. Mechanistically, circPUM1 upregulates the expression of RAB27B and VEGFA by sponging miR-607, thus boosting release of exosome facilitated by RAB27B and directly activating VEGF signaling in endothelial cells to foster angiogenesis. To disrupt this circPUM1-driven TME modulation, we engineered an innovative pH-responsive 2D niobium carbide nanosheets loaded with circPUM1 siRNA. Through excessive PEI grafting, we functionalized the nanosheets with cationic property, achieving efficient negative-charged siRNA loading. Further surface PEGylation shielded the nanosheets’ positive charge, reducing off-target effect and systemic toxicity, while acidic TME triggered PEG exfoliation for tumor-specific circPUM1 siRNA delivery. Functional cellular assays and an intraperitoneal tumor-bearing mouse model validated that the nanosheet-delivered circPUM1 siRNA effectively inhibited angiogenesis and peritoneal dissemination by knocking down circPUM1 expression and subsequently downregulating its downstream targets. This study uncovers a novel exosome-mediated angiogenesis mechanism and develops innovative MXene nanosheets for pH-responsive siRNA delivery providing a promising strategy for ovarian cancer precision therapy with significant clinical translational value and application potential.
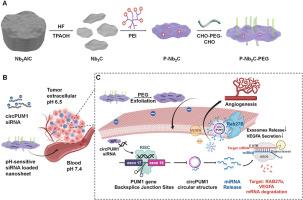
ph响应二维碳化铌纳米片靶向circPUM1 siRNA递送卵巢癌治疗
卵巢癌的进展与肿瘤微环境(TME)失调密切相关,特别是由外泌体介导的串扰驱动的病理性血管生成。在这里,我们阐明了卵巢癌来源的circPUM1通过外泌体转移到血管内皮细胞来促进血管生成。在机制上,circPUM1通过海绵化miR-607上调RAB27B和VEGFA的表达,从而促进RAB27B促进外泌体的释放,直接激活内皮细胞中的VEGF信号,促进血管生成。为了破坏这种circPUM1驱动的TME调制,我们设计了一种负载circPUM1 siRNA的创新型ph响应2D碳化铌纳米片。通过过量的PEI接枝,我们将纳米片功能化为具有阳离子性质,实现了高效的负电荷siRNA负载。进一步的表面聚乙二醇化屏蔽了纳米片的正电荷,减少了脱靶效应和全身毒性,而酸性TME则触发了肿瘤特异性circPUM1 siRNA递送的聚乙二醇脱落。功能细胞实验和腹腔肿瘤小鼠模型证实,纳米薄片递送的circPUM1 siRNA通过降低circPUM1表达并随后下调其下游靶点,有效抑制血管生成和腹膜播散。本研究揭示了一种新的外泌体介导的血管生成机制,并开发了用于ph响应siRNA递送的创新型MXene纳米片,为卵巢癌精确治疗提供了一种有前景的策略,具有重要的临床转化价值和应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


