Realization of differential release of minocycline hydrochloride from electrosprayed polymeric or biomacromolecular microparticles for the repair of traumatic brain injury
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Traumatic brain injury (TBI) occurs when an external force impacts the brain and can result in various serious consequences. Currently, there are no clinical therapies to satisfy the different pathophysiological stages of TBI, realizing a combination of anti-inflammatories in the acute stage and polarization of M2 phenotype microglia. Herein, poly(lactic-co-glycolic acid) (PLGA) and silk fibroin (SF) were selected as shell layers, respectively, to fabricate minocycline hydrochloride (MH)-loaded core-shell microparticles through coaxial electrospray. MH@SF and MH@PLGA microparticles exhibited a differential release profile to promote nerve repair and regeneration after TBI. MH@SF achieved a fast release in 7 days to suppress neuroinflammatory response, while the sustained release of MH@PLGA up to 25 days allowed for modulating polarization of M2 phenotype microglia. The obtained microparticles promoted cell viability and neurite growth of primary neurons and SH-SY5Y cells by establishing neurite transection (NT) and oxygen-glucose deprivation (OGD) models to simulate primary and secondary injury after TBI. In vivo experiments further proved that MH@SF and MH@PLGA microparticles alleviate the neuroinflammation microenvironment and enhance motor, learning, and memory functions of mice after TBI. In summary, this work proposed a promising strategy to regulate the neuroimmune microenvironment through matching different pathophysiological stages of TBI, demonstrating potential for clinical translation to treat TBI.
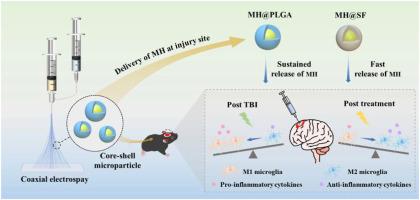
电喷涂高分子微粒与生物大分子微粒对盐酸米诺环素差异释放修复脑外伤的研究
外伤性脑损伤(TBI)发生在外力冲击大脑时,可导致各种严重后果。目前尚无临床治疗方法能够满足TBI的不同病理生理阶段,实现急性期抗炎与M2表型小胶质细胞极化相结合。本文以聚乳酸-羟基乙酸(PLGA)和丝素(SF)为壳层,采用同轴电喷雾法制备了负载米诺环素(MH)的核壳微粒子。MH@SF和MH@PLGA微颗粒在促进脑损伤后神经修复和再生方面表现出不同的释放特征。MH@SF在7天内实现快速释放以抑制神经炎症反应,而MH@PLGA的持续释放长达25天,可调节M2表型小胶质细胞的极化。通过建立神经突横断(NT)和氧-葡萄糖剥夺(OGD)模型模拟脑外伤后原发性和继发性损伤,获得的微颗粒促进了原代神经元和SH-SY5Y细胞的细胞活力和神经突生长。体内实验进一步证明MH@SF和MH@PLGA微颗粒可以缓解脑外伤后小鼠的神经炎症微环境,增强小鼠的运动、学习和记忆功能。综上所述,本研究提出了一种有前景的策略,通过匹配TBI的不同病理生理阶段来调节神经免疫微环境,显示了临床转化治疗TBI的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


