3D-printed nHA/PA66 porous scaffold: Regulating immune balance and vascularization synergistically promotes bone regeneration
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
3D-printed porous scaffolds have emerged as a potential solution for treating critical-sized bone defects, yet localized inflammatory imbalance leads to suboptimal therapeutic outcomes. This study utilized nHA/PA66 composite material as the foundation, employing APF additive manufacturing technology to fabricate a three-dimensional porous scaffold (HP) suitable for local bone filling, and functionalized the porous scaffold through modification with polydopamine and magnesium ions (Mg2+) (HPDM). First, the physicochemical properties and biosafety of the HPDM scaffold were verified, followed by a comparative analysis of its effects on macrophage phenotype transformation, vascular endothelial cell differentiation, and pre-osteoblast differentiation differences. The HPDM scaffold achieves coordinated balance in the transformation between M1 and M2 macrophages through sustained release of polydopamine and Mg2+. Mg2+ plays a crucial role in inflammatory regulation by downregulating the NF-κB signaling pathway. Furthermore, several experiments demonstrated that the HPDM scaffold regulates orderly inflammatory responses to promote intercellular interactions, stimulating angiogenesis and osteogenic regeneration. In New Zealand rabbits' femoral condyle bone defect model, the HPDM porous scaffold achieved significant vascularized bone regeneration. This study confirms that the functionalized HPDM porous scaffold prepared using nHA/PA66 as the base material has significant potential in regulating immune responses and enhancing vascularized bone regeneration.
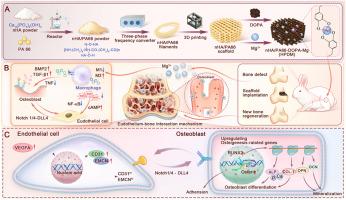
3d打印nHA/PA66多孔支架:调节免疫平衡和血管化协同促进骨再生
3d打印多孔支架已成为治疗临界尺寸骨缺损的潜在解决方案,但局部炎症不平衡导致治疗效果不理想。本研究以nHA/PA66复合材料为基础,采用APF增材制造技术制备出适合局部骨填充的三维多孔支架(HP),并通过聚多巴胺和镁离子(Mg2+) (HPDM)改性实现多孔支架的功能化。首先验证HPDM支架的理化性质和生物安全性,然后比较分析其对巨噬细胞表型转化、血管内皮细胞分化和成骨前细胞分化差异的影响。HPDM支架通过持续释放多多巴胺和Mg2+,实现巨噬细胞M1和M2转化的协调平衡。Mg2+通过下调NF-κB信号通路在炎症调节中发挥重要作用。此外,一些实验表明,HPDM支架调节有序的炎症反应,促进细胞间相互作用,刺激血管生成和成骨再生。在新西兰兔股骨髁骨缺损模型中,HPDM多孔支架实现了明显的血管化骨再生。本研究证实了以nHA/PA66为基础材料制备的功能化HPDM多孔支架在调节免疫反应和增强血管化骨再生方面具有重要的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


