Recent advances in metal ions overloading for tumors: Mechanisms, nanomaterials, and therapies
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Metal ions serve as indispensable regulators in fundamental biological processes, maintaining critical physiological functions including osmotic equilibrium, acid-base regulation, intracellular signaling, and biomolecular recognition. Perturbations of ionic homeostasis can disrupt cellular integrity, leading to functional impairment and ultimately programmed cell death. Capitalizing on this paradigm, emerging nanotherapeutic approaches have pioneered the strategic induction of tumor-selective ions overloading as a potent anticancer strategy. This comprehensive review critically evaluates the roles of key metal ions (Na+, K+, Ca2+, Cu2+, Zn2+, Fe2+/3+, Mn2+) in tumor progression and their mechanisms of action when overaccumulated, and highlights innovative nanomaterial designs that exploit ions overloading to induce apoptosis, pyroptosis, or immunogenic cell death. Meanwhile, the combinatorial approaches integrating ions-overloading with other therapy including immunotherapy, chemodynamic therapy et al. would be discussed. By integrating the mechanisms and contemporary research advances, this work provides a conceptual framework for developing next-generation of ions-disrupting nanomedicines and identifies promising directions for combinatorial anticancer regimens.
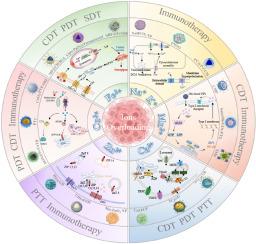
肿瘤金属离子超载的最新进展:机制、纳米材料和治疗
金属离子在基本的生物过程中是必不可少的调节剂,维持重要的生理功能,包括渗透平衡、酸碱调节、细胞内信号传导和生物分子识别。离子稳态的扰动会破坏细胞完整性,导致功能损伤,最终导致程序性细胞死亡。利用这一范例,新兴的纳米治疗方法开创了诱导肿瘤选择性离子过载的策略,作为一种有效的抗癌策略。这篇全面的综述批判性地评估了关键金属离子(Na+、K+、Ca2+、Cu2+、Zn2+、Fe2+/3+、Mn2+)在肿瘤进展中的作用及其过度积累时的作用机制,并强调了利用离子过载诱导细胞凋亡、焦亡或免疫原性细胞死亡的创新纳米材料设计。同时,还将讨论离子超载与免疫治疗、化学动力治疗等其他治疗方法的组合方法。通过整合机制和当代研究进展,本工作为开发下一代离子破坏纳米药物提供了一个概念框架,并为联合抗癌方案确定了有希望的方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


