Structural studies of Cs2AgBiBr6 double perovskite
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The structure of Cs2AgBiBr6 between 80 and 350 K was determined by Rietveld refinement of high-resolution synchrotron X-ray diffraction data. At room temperature, the material adopts a cubic Fm m structure with rock-salt-like ordering of Ag+ and Bi3+ cations, doubling the perovskite unit cell. Despite the lower valence Ag+ being larger than Bi3+ (1.15 vs 1.03 Å), the Ag–Br bond is slightly shorter than the Bi–Br bond (2.8103(8) vs 2.8229(8) Å), with nearly equal MO6 octahedral volumes, suggesting greater covalency and distortion in AgBr6 units. The Cs+ cations are underbonded in the cubic phase and large anisotropic Br displacements perpendicular to Ag–Br–Bi chains, attributed to dynamic rotations of corner-sharing octahedra, partially offsetting this underbonding. Below 120 K, the Br displacements freeze, inducing a continuous transition to a tetragonal I4/m structure. An unusual expansion in the volume of the BiBr6 polyhedara occurs in the low temperature tetragonal phase. The structural changes with temperature reflect the balance between ionic size, bonding character, and dynamic lattice effects.
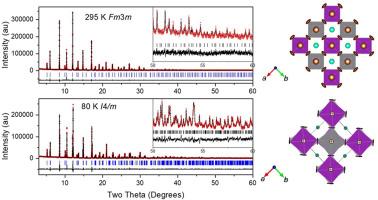
Cs2AgBiBr6双钙钛矿的结构研究
采用高分辨率同步加速器x射线衍射数据的Rietveld细化,确定了Cs2AgBiBr6在80 ~ 350 K之间的结构。在室温下,材料采用立方f3m形式,以岩盐形式排列Ag+和Bi3+阳离子,使钙钛矿单元电池加倍。尽管价态较低的Ag+比Bi3+大(1.15 vs 1.03 Å), Ag - br键比Bi-Br键略短(2.8103(8)vs 2.8229(8) Å), MO6八面体体积几乎相等,表明AgBr6单元的共价和畸变更大。Cs+阳离子在立方相中存在欠键,而垂直于Ag-Br-Bi链的大的各向异性Br位移,归因于共享角八面体的动态旋转,部分抵消了这种欠键。当温度低于120k时,Br的位移冻结,导致其连续转变为I4/m的四边形结构。在低温四方相中,BiBr6多面体的体积出现了不寻常的膨胀。结构随温度的变化反映了离子大小、成键特性和动态晶格效应之间的平衡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


