Mixed modifier effect in Na2O–BaO–B2O3 glasses: Structure-forming role of alkali-alkaline earth combinations
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
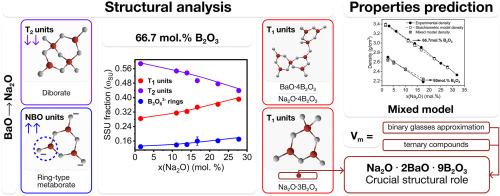
This study investigates the mixed modifier effect on structure in Na2O–BaO–B2O3 glasses, driven by variations in the modifier ratio (Na2O/BaO) across different glass-forming regions (B2O3 concentrations). Vibrational spectroscopy methods (IR and Raman) were used to analyze the short-range order (expressed as the fraction of tetrahedral boron, N4) and the intermediate-range order (represented by superstructural units) structure.
The results reveal a near-linear decrease in N4 and a higher concentration of asymmetric (metaborate) triangles in more alkaline glasses which is particularly pronounced in the most modified series (33.3 mol.% modifiers). In glasses with low modifier content (≤20 mol.%), the fraction of superstructural units with one tetrahedron (T1) exhibits nonlinear behavior, whereas in the high-modified series (33.3 mol.%), this dependence follows a linear trend, showing a steady increase in T1 units’ fraction with increasing Na2O content.
Structural analysis of Na2O–BaO–B2O3 glasses revealed that the structural effects observed during BaO/Na2O substitution in three glass series are primarily governed by two key factors: the preferential formation of mixed boron-oxygen network anions (stoichiometric analogues of multicomponent crystalline compounds) and the phase diversity of crystalline compounds in the binary Na2O–B2O3 and BaO–B2O3 systems. These findings challenge the conventional approach of considering multicomponent borate glass structures as simple combinations of binary subnetworks.
The structural calculations were supported by density and molar refractivity modelling for both low- and high-modified glass series. The calculated density values agreed well with experimental data, showing deviations comparable to those obtained in high-accuracy thermodynamic calculations.
混合改性剂在na20 - bao - b2o3玻璃中的作用:碱-碱土组合的结构形成作用
本研究考察了混合改性剂对Na2O - BaO - B2O3玻璃结构的影响,通过不同玻璃形成区域(B2O3浓度)的改性剂比(Na2O/BaO)的变化来驱动。采用红外光谱和拉曼光谱等振动光谱方法分析了其近阶结构(以四面体硼N4的分数表示)和中阶结构(以上层结构单元表示)。结果表明,在碱性较强的玻璃中,N4含量呈近线性下降,不对称(偏酸盐)三角形的浓度较高,这在改性最多的玻璃系列(改性剂为33.3%)中尤为明显。在改性剂含量低(≤20mol .%)的玻璃中,具有一个四面体(T1)的上层结构单元的比例呈现非线性行为,而在高改性系列(33.3 mol.%)中,这种依赖关系遵循线性趋势,随着Na2O含量的增加,T1单元的比例稳步增加。对na20 - BaO - b2o3玻璃的结构分析表明,三个玻璃系列在BaO/Na2O取代过程中所观察到的结构效应主要由两个关键因素决定:混合硼氧网络阴离子(多组分晶体化合物的化学计量类似物)的优先形成以及二元na20 - b2o3和BaO - b2o3体系中晶体化合物的物相多样性。这些发现挑战了将多组分硼酸盐玻璃结构视为二元子网的简单组合的传统方法。结构计算得到了密度和摩尔折射率模型对低和高改性玻璃系列的支持。计算的密度值与实验数据吻合得很好,其偏差与高精度热力学计算结果相当。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


