Investigating rapid alpha-decay induced aging of 238PuO2 by Raman Spectroscopy
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
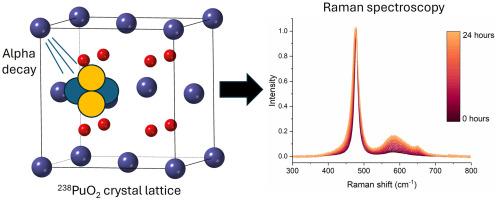
Investigating alpha-decay induced aging in PuO2 is useful in nuclear forensics; helping to determine the time since last calcination. This was previously investigate by monitoring the damage to 240PuO2 over the course of a few years. The rate of alpha-decay induced aging has long been assumed to scale only with the decay rate of other isotopes without direct verification. This article reports the first alpha-decay aging study of 238PuO2 by Raman spectroscopy. Contrary to the expected 10 days for 238PuO2 to reach steady state based on 240PuO2 studies, the alpha aging curve reached an approximate steady state ∼24–30 h after laser annealing. While the cause of the order of magnitude decrease is unknown, it is speculated that dynamic annealing could contribute to the differences in rate of induced aging. The Raman spectra of annealed and aged 238PuO2 matched the expected features in 239PuO2 and 240PuO2 spectra; showing no formation of new stable chemical species in the material such as secondary Pu oxide phases (e.g., Pu4O9). Results indicate that 238Pu could be leveraged for rapid alpha-decay aging studies to characterize alpha-decay induced features and better understand matrix temperatures and the annealing of Frankel pair defects.
用拉曼光谱研究238PuO2的快速α衰变老化
研究PuO2中α衰变引起的老化在核取证中是有用的;帮助确定上次煅烧后的时间。之前的研究是在几年的时间里通过监测对240PuO2的损害来进行的。长期以来,人们一直认为α衰变诱发老化的速率只与其他同位素的衰变速率成比例,没有得到直接验证。本文报道了利用拉曼光谱对238PuO2进行α衰变老化的首次研究。与基于240PuO2研究的238PuO2达到稳态所需的10天不同,激光退火后α老化曲线达到近似稳态~ 24-30 h。虽然数量级下降的原因尚不清楚,但推测动态退火可能导致诱导时效率的差异。退火和时效后的238PuO2的拉曼光谱符合239PuO2和240PuO2的光谱特征;在材料中没有形成新的稳定的化学物质,如二次Pu氧化物相(如Pu4O9)。结果表明,238Pu可以用于快速α衰变老化研究,以表征α衰变诱导的特征,并更好地了解基体温度和Frankel对缺陷的退火。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


