Performance evaluation and mechanistic insights into melanoidin adsorption and photodegradation by PMoV-X@101(Fe)
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-09-13
DOI:10.1016/j.jphotochem.2025.116785
引用次数: 0
Abstract
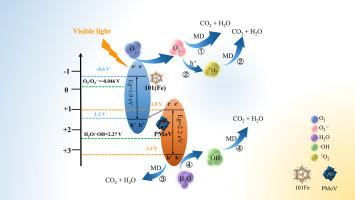
To address the substantial presence of melanoidin (MD) in wastewater, a Z-scheme heterojunction photocatalyst, PMoV-X@101(Fe), was synthesized using a metal-organic framework (101(Fe)) and polyoxometalate (H5PMo10V2O40, PMoV) via an in situ hydrothermal method. Subsequently, a systematic study was carried out to assess the adsorption performance of PMoV-X@101(Fe)for MD, including its adsorption capacity, isotherms, kinetics, and underlying mechanisms. In addition, the degradation performance of PMoV-X@101(Fe) was also assessed by analyzing the degradation kinetics, the reactive species involved, and their interactions. The results showed that PMoV-X@101(Fe) exhibited an exceptional adsorption capacity of 450 mg/g, driven primarily by electrostatic interactions and hydrogen bonding. Furthermore, under visible light irradiation for 40 min, PMoV-0.2@101(Fe) achieved a 90.2 % degradation rate of a 500 mg/L MD solution. The dominant reactive species, O2⋅− and h+, synergistically interacted with ⋅OH and 1O2, In addition to these findings, the influence of coexisting ions on MD degradation, along with toxicity assessments, recyclability, and catalyst stability, was also explored. Compared with previously reported materials, PMoV-X@101(Fe) independently provides both high adsorption and high photocatalytic activity, while treated MD solutions exhibited reduced toxicity, demonstrating environmental safety. Overall, PMoV-X@101(Fe) demonstrated dual-function efficiency, robust stability, and practical applicability, offering a comprehensive strategy for MD removal and addressing critical gaps in wastewater treatment research.
PMoV-X@101(Fe)吸附和光降解类黑素的性能评价及机理研究
为了解决废水中大量存在的类黑素(MD)问题,以金属有机骨架(101(Fe))和多金属氧酸盐(H5PMo10V2O40, PMoV)为原料,采用原位水热法制备了z型异质结光催化剂PMoV-X@101(Fe)。随后,进行了系统的研究,以评估PMoV-X@101(Fe)对MD的吸附性能,包括其吸附容量,等温线,动力学和潜在机制。此外,还通过分析降解动力学、所涉及的反应物质及其相互作用来评价PMoV-X@101(Fe)的降解性能。结果表明,PMoV-X@101(Fe)的吸附能力为450 mg/g,主要由静电相互作用和氢键驱动。此外,在可见光照射40分钟下,PMoV-0.2@101(Fe)对500 mg/L MD溶液的降解率达到90.2%。O2⋅−和h+与⋅OH和1O2具有协同作用。此外,还探讨了共存离子对MD降解、毒性评估、可回收性和催化剂稳定性的影响。与先前报道的材料相比,PMoV-X@101(Fe)独立提供了高吸附和光催化活性,而处理过的MD溶液毒性降低,证明了环境安全性。总体而言,PMoV-X@101(Fe)展示了双重功能效率,强大的稳定性和实用性,为去除MD提供了全面的策略,并解决了废水处理研究中的关键空白。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


