Noncovalent complexes of anionic carbocyanine dyes with human serum albumin as a basis for turn-off fluorescence sensors for bilirubin. Effect of Cu2+ ions
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-09-12
DOI:10.1016/j.jphotochem.2025.116781
引用次数: 0
Abstract
The development of highly sensitive and selective turn-off fluorescence sensors for bilirubin (BR) is of significant clinical importance. This work explores the basis for such sensors using the noncovalent complexes of meso-substituted anionic carbocyanine dyes with human serum albumin (HSA). The binding of these dyes to HSA is characterized by high association constants (of the order of 105–106 M−1) and induces a pronounced fluorescence increase due to the stabilization of monomeric trans-isomers. Subsequent introduction of BR, which itself binds strongly to HSA, results in efficient fluorescence quenching (super-quenching effect). Comprehensive spectral studies and molecular docking simulations indicate that the primary quenching mechanism is the competitive displacement of dye molecules from the complex with HSA into the weakly fluorescent free state. The quenching effect is somewhat enhanced in the presence of Cu2+ ions. The effective Stern–Volmer quenching constants were determined, and the analytical potential of these systems was evaluated, revealing low limits of detection (LOD) and quantification (LOQ) of BR. The dye–HSA and dye–HSA–Cu2+ complexes presented herein thus create promising and efficient platforms for the rational design of turn-off sensors for BR.
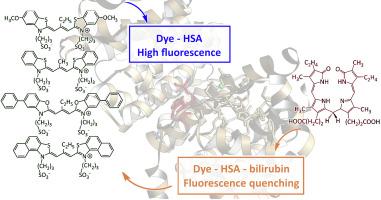
阴离子碳菁染料与人血清白蛋白的非共价配合物作为关闭胆红素荧光传感器的基础。Cu2+离子的影响
开发高灵敏度、高选择性的胆红素关闭荧光传感器具有重要的临床意义。这项工作探讨了使用中位取代阴离子碳菁染料与人血清白蛋白(HSA)的非共价配合物的这种传感器的基础。这些染料与HSA的结合具有高缔合常数(约105-106 M−1)的特征,并且由于单体反式异构体的稳定而引起明显的荧光增强。随后引入BR, BR本身与HSA强结合,导致有效的荧光猝灭(超猝灭效应)。综合光谱研究和分子对接模拟表明,主要的猝灭机制是染料分子从与HSA配合物竞争位移到弱荧光自由态。Cu2+离子的存在使淬火效果有所增强。测定了有效的Stern-Volmer猝灭常数,并评价了这些体系的分析潜力,揭示了BR的低检出限(LOD)和定量限(LOQ)。因此,本文提出的染料- hsa和染料- hsa - cu2 +配合物为合理设计BR关断传感器提供了有前途和有效的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


