Substituent effects on solid-state emission of 2,7,9-triphenylcarbazoles
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-09-16
DOI:10.1016/j.jphotochem.2025.116794
引用次数: 0
Abstract
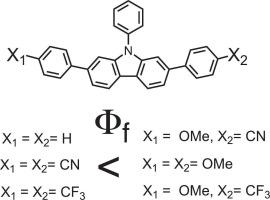
Previously, we reported polycyclic aromatic hydrocarbons with multiple chromophores exhibit solid-state emission. We report synthesis and photophysical characterization of 2,7,9-triphenyl carbazole derivatives in which the phenyl rings at the 2- and 7-positions are selectively substituted with electron-donating and electron-withdrawing groups. The effects of these substituents on the emission properties were systematically investigated in solution and the solid state. The incorporation of electron-donating groups significantly enhanced the emission properties whereas electron-withdrawing groups had minimal impact. Density functional theory (DFT) calculations revealed that the highest occupied molecular orbitals (HOMOs) are predominantly localized on the carbazole core and electron-rich phenyl rings, while the lowest unoccupied molecular orbitals (LUMOs) are delocalized over the chromophore framework excluding the 9-phenyl moiety. Overall, the present study demonstrates a rational molecular design strategy for enhancing solid-state emission through peripheral electronic tuning of carbazole-based luminophores, with potential implications for the development of efficient organic light-emitting materials.
取代基对2,7,9-三苯基咔唑固态发射的影响
以前,我们报道了具有多个发色团的多环芳烃表现出固态发射。本文报道了2,7,9-三苯基咔唑衍生物的合成和光物理性质,其中2位和7位的苯环被选择性地取代为供电子和吸电子基团。系统地研究了这些取代基在溶液和固体状态下对发射性能的影响。给电子基团的加入显著提高了材料的发射性能,而吸电子基团的加入对材料的发射性能影响很小。密度泛函理论(DFT)计算表明,最高占位分子轨道(HOMOs)主要定位在carbazole核心和富电子苯基环上,而最低未占位分子轨道(LUMOs)则在除9-苯基部分外的发色团框架上离域。总的来说,本研究展示了一种合理的分子设计策略,可以通过咔唑基发光团的外围电子调谐来增强固态发光,这对开发高效有机发光材料具有潜在的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


