Hydrothermal hydrodenitrogenation and hydrodesulfurization over nickel-based catalysts in sub/supercritical water
IF 7.7
2区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
As an inherent by-product of the petroleum industry, oily sludge has substantial potential as energy resource due to its high oil content and calorific value. The hydrodenitrogenation/hydrodesulfurization of nitrogen/sulfur compounds quinoline and thiophene in oily sludge at sub/supercritical hydrothermal conditions was studied, along with its catalytic properties and reaction mechanism. The effects of nickel-based catalysts, hydrogen sources, and reaction conditions on hydrodenitrogenation (HDN) of quinoline were examined. A reaction kinetic model for quinoline in supercritical water was developed. An experimental investigation on hydrodesulfurization (HDS) of sulfur-containing thiophene was conducted, and the catalytic properties of nickel-based catalysts for simultaneous HDN and HDS of quinoline and thiophene were evaluated. Within 400–440 °C, ethanol was superior to formic acid as hydrogen source. Ni-Co/γ-Al2O3 had the most effective catalytic impact on denitrogenation of quinoline. The conversion efficiency of 5 wt% quinoline reached 94.67 %, while denitrogenation efficiency was 57.08 % at 24 MPa, 440 °C, and 60 min. The hydrogenation and ring-opening steps had significant effects on the overall denitrogenation process. The hydrodesulfurization catalysis from Ni-Mo/γ-Al2O3 was the most prominent for thiophene. At 24 MPa, 440 °C, and 60 min, the desulfurization efficiency of thiophene reached 57.34 %. Desulfurization of thiophene mainly followed the hydrogenation route, with thiophene rings being saturated before desulfurization occurred.
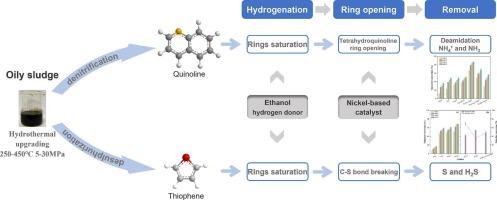
亚/超临界水中镍基催化剂的水热加氢脱氮和加氢脱硫
含油污泥作为石油工业的固有副产品,因其含油量高、热值高,具有巨大的能源潜力。研究了亚/超临界水热条件下含氮/含硫化合物喹啉和噻吩在含油污泥中的加氢脱氮/加氢脱硫及其催化性能和反应机理。考察了镍基催化剂、氢源和反应条件对喹啉加氢脱氮反应的影响。建立了喹啉在超临界水中的反应动力学模型。对含硫噻吩的加氢脱硫(HDS)进行了实验研究,并评价了镍基催化剂对喹啉和噻吩同时加氢脱硫和加氢脱硫的催化性能。在400 ~ 440℃范围内,乙醇作为氢源优于甲酸。Ni-Co/γ-Al2O3对喹啉脱氮的催化效果最好。在24 MPa、440℃、60 min条件下,5 wt%喹啉的转化率为94.67%,脱氮效率为57.08%。加氢和开环步骤对整个脱氮过程有显著影响。Ni-Mo/γ-Al2O3对噻吩的加氢脱硫催化作用最为显著。在24 MPa、440℃、60 min条件下,噻吩的脱硫效率可达57.34%。噻吩的脱硫主要采用加氢途径,脱硫前噻吩环被饱和。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel Processing Technology
工程技术-工程:化工
CiteScore
13.20
自引率
9.30%
发文量
398
审稿时长
26 days
期刊介绍:
Fuel Processing Technology (FPT) deals with the scientific and technological aspects of converting fossil and renewable resources to clean fuels, value-added chemicals, fuel-related advanced carbon materials and by-products. In addition to the traditional non-nuclear fossil fuels, biomass and wastes, papers on the integration of renewables such as solar and wind energy and energy storage into the fuel processing processes, as well as papers on the production and conversion of non-carbon-containing fuels such as hydrogen and ammonia, are also welcome. While chemical conversion is emphasized, papers on advanced physical conversion processes are also considered for publication in FPT. Papers on the fundamental aspects of fuel structure and properties will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


