High-efficiency lithium recovery from desalination brine via MnAl layered double hydroxide
IF 9.8
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
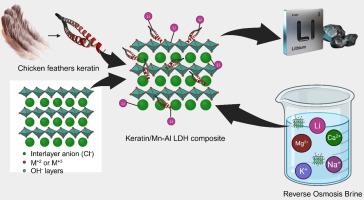
The increasing global population necessitates the exploration and adoption of environmentally friendly extraction methods and alternative sources to meet the growing demand for lithium (Li). Adsorption offers high selectivity for Li and has emerged as a promising and cost-effective alternative. However, current methods utilizing manganese- and titanium-based adsorbents present environmental risks and exhibit instability under acidic conditions. Layered double hydroxides (LDHs) show significant potential for Li extraction from various sources, including brine. This study aimed to synthesize a novel manganese‑aluminum double-layered hydroxide (Mn![]() Al LDH) combined with keratin for Li extraction from brine. The Keratin/Mn-Al LDH demonstrated efficient Li adsorption with a capacity of 49.7 mg/g and a desorption rate of 92 %. Both the Langmuir and Temkin isotherms effectively represented the equilibrium data, indicating monolayer adsorption with chemisorption characteristics. Moreover, the Pseudo-Second-Order (PSO) kinetic model accurately depicted the adsorption rate, suggesting that chemical interactions predominantly govern the process. Furthermore, the maximum adsorption capacity, as determined by the Langmuir model, was 384.62 mg/g. These results suggest a promising direction for exploring the potential of LDH in Li extraction.
Al LDH) combined with keratin for Li extraction from brine. The Keratin/Mn-Al LDH demonstrated efficient Li adsorption with a capacity of 49.7 mg/g and a desorption rate of 92 %. Both the Langmuir and Temkin isotherms effectively represented the equilibrium data, indicating monolayer adsorption with chemisorption characteristics. Moreover, the Pseudo-Second-Order (PSO) kinetic model accurately depicted the adsorption rate, suggesting that chemical interactions predominantly govern the process. Furthermore, the maximum adsorption capacity, as determined by the Langmuir model, was 384.62 mg/g. These results suggest a promising direction for exploring the potential of LDH in Li extraction.
MnAl层状双氢氧化物从海水淡化盐水中高效回收锂
随着全球人口的不断增长,需要探索和采用环保的提取方法和替代来源,以满足对锂(Li)日益增长的需求。吸附对Li具有高选择性,已成为一种有前途且具有成本效益的替代方法。然而,目前使用锰基和钛基吸附剂的方法存在环境风险,并且在酸性条件下表现出不稳定性。层状双氢氧化物(LDHs)具有从包括卤水在内的各种来源提取锂的巨大潜力。本研究旨在合成一种新型的锰铝双层氢氧化物(MnAl - LDH)与角蛋白结合,用于从盐水中提取锂。角蛋白/Mn-Al LDH对Li的吸附效率为49.7 mg/g,解吸率为92%。Langmuir等温线和Temkin等温线均有效表征了平衡数据,表明了具有化学吸附特征的单层吸附。此外,伪二阶(PSO)动力学模型准确地描述了吸附速率,表明化学相互作用主导了这一过程。Langmuir模型测定的最大吸附量为384.62 mg/g。这些结果为探索LDH在锂提取中的潜力提供了一个有希望的方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


