Sustainable access to novel pyridone scaffolds: Efficient one-pot, three-component synthesis in PEG-400
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
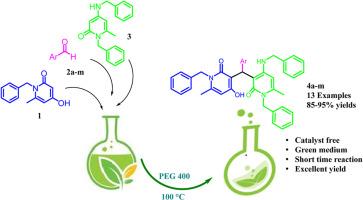
This study presents a straightforward and efficient method for synthesizing a novel series of 1-benzyl-3-((1-benzyl-4-(benzylamino)-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)(aryl)methyl)-4-hydroxy-6-methylpyridin-2(1H)-one derivatives through a one-pot, three-component reaction. The process employs 1-benzyl-4-hydroxy-6-methylpyridin-2(1H)-one, diverse aromatic aldehydes, and 1-benzyl-4-(benzylamino)-6-methylpyridin-2(1H)-one in polyethylene glycol (PEG-400) as an environmentally benign solvent. The methodology prioritizes efficiency, scalability, and reduced environmental impact, offering a practical route to functionalized pyridinones.
We report a catalyst-free protocol for the efficient synthesis of biologically relevant heterocycles, notably functionalized pyridinones. This method features high atom economy, broad functional group tolerance, and minimal purification steps, embodying the sustainable chemistry principles. All products were characterized by multi-spectroscopic analyses (1H/13C NMR, IR, MS) and elemental analysis. Furthermore, single-crystal X-ray diffraction provided unambiguous structural confirmation for a representative derivative.
新型吡啶酮支架的可持续获取:PEG-400中高效的一锅三组分合成
本研究提出了一种简单有效的方法,通过一锅三组分反应合成了一系列新的1-苄基-3-((1-苄基-4-(苄基氨基))-6-甲基-2-氧-1,2-二氢吡啶-3-基)(芳基)甲基)-4-羟基-6-甲基吡啶-2(1H)- 1衍生物。该工艺采用1-苄基-4-羟基-6-甲基吡啶-2(1H)- 1,多种芳香醛和1-苄基-4-(苄基氨基)-6-甲基吡啶-2(1H)- 1在聚乙二醇(PEG-400)中作为环保溶剂。该方法优先考虑效率、可扩展性和减少环境影响,为功能化吡啶酮提供了一条实用的途径。我们报道了一种无催化剂的方案,用于有效合成生物相关的杂环,特别是功能化吡啶酮。该方法具有原子经济性高、官能团耐受性广、纯化步骤少等特点,体现了可持续化学原理。通过多光谱分析(1H/13C NMR、IR、MS)和元素分析对产物进行了表征。此外,单晶x射线衍射为具有代表性的导数提供了明确的结构确认。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


