Amidation of amino aryl acids with orthoesters in aqueous ammonium hydroxide: A strategy to construct quinazolinones and pyrido[2,3-d]pyrimidinones
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A one-pot oxidative cyclization of 2-amino aryl acids with orthoesters to quinazoline-4(3H)-ones and pyrido[2,3-d]pyrimidinones was described in a greener approach using aqueous ammonium hydroxide. The synthetic protocol enables greater selectivity with synthetic utility. A wide range of substrate scope was explored with good to excellent yields. The reaction mechanism involves N-benzylation/alkylation, amidation, and subsequent oxidative annulation.
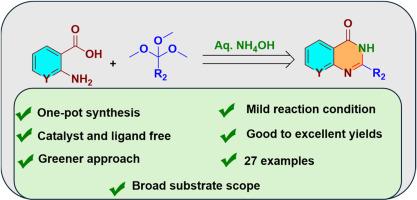
氨基芳基酸与正位酯在氢氧化铵水溶液中的酰胺化:一种构建喹唑啉酮和吡啶[2,3-d]嘧啶酮的策略
采用一种更环保的方法,用氢氧化铵水溶液将2-氨基芳基酸与邻苯二甲酸酯一锅氧化环化成喹唑啉-4(3H)酮和吡啶[2,3-d]嘧啶酮。合成协议使合成工具具有更大的选择性。探索了广泛的衬底范围,并获得了良好的收率。反应机理包括n -苄基化/烷基化、酰胺化和随后的氧化环化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


