Synthesis of 1,3-dialkylthioselenoglycolurils - novel antifungal agents
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A two-step synthesis of pharmacologically relevant 1,3-dialkyl-5-selenoxohexahydroimidazo[4,5-d]imidazole-2(1H)-thiones (thioselenoglycolurils) starting from readily available thioglycolurils was realized for the first time. The developed protocol involves S-methylation of initial thioglycolurils followed by selenation of thus formed isothiouronium salts with NaHSe generated in situ from grey Se and NaBH4. In vitro study of antifungal activity against Sclerotinia sclerotiorum, Fusarium oxysporum, Fusarium maniliforme, Bipolaris sorokiniana, Venturia inaequalis, Rhizoctonia solani phytopathogens and yeasts Candida albicans and Cryptococcus neoformans, Candida auris, MDR Candida auris, Candida glabrata was performed. It was found that 8 thioselenoglycolurils completely inhibited the growth of V. inaequalis and R. solani fungi and partially suppress the growth of B. sorokiniana fungus (MGI 59–67 %) analogous to triadimefon (MGI 68 %). Effective antifungal action towards C. albicans and C. neoformans (MIC 0.25 μg/mL) with no discernible toxicity against mamallian cells was revealed for 4 representatives of thioselenoglycolurils. Effective growth inhibition of gram-positive bacterium S. aureus, yeast C. neoformans, C. auris, MDR C. auris, C. glabrata was additionally noted for 1,3-diethylthioselenoglycoluril. Moreover, the latter compound has low toxicity (LD50 = 129 mg/kg) and can be referred to as a lead candidate for the development of new antifungal pharmaceuticals.
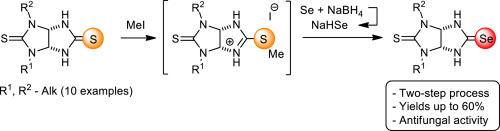
新型抗真菌剂1,3-二烷基硫代硒代糖苷的合成
首次实现了以易得的硫代糖基化合物为原料,两步法合成具有药理意义的1,3-二烷基-5-硒氧六氢咪唑[4,5-d]咪唑-2(1H)-硫代糖基化合物(硫代硒代糖基化合物)。开发的方案包括初始巯基基的s甲基化,然后用灰硒和NaBH4原位生成的NaHSe将由此形成的异硫脲盐硒化。体外研究了其对菌核菌核菌、尖孢镰刀菌、梨形镰刀菌、双极镰刀菌、不均匀文氏菌、茄根丝胞菌、白色念珠菌和新型隐球菌、耳念珠菌、耐多药耳念珠菌、光秃念珠菌的抑菌活性。结果表明,8硫代硒代糖苷完全抑制不平等弧菌(V. inaequalis)和番茄枯萎菌(R. solani)的生长,部分抑制sorokiniana真菌(MGI为59 ~ 67%)的生长,类似于三啶酮(MGI为68%)。结果表明,4种硫代硒酸糖化合物对白色念珠菌和新生念珠菌均有较好的抗真菌作用(MIC≤0.25 μg/mL),对哺乳动物细胞无明显毒性。此外,1,3-二乙基硫代硒代甘醇还能有效抑制革兰氏阳性细菌金黄色葡萄球菌、酵母菌新生酵母、金黄色葡萄球菌、耐多药金黄色葡萄球菌、光秃葡萄球菌的生长。此外,后一种化合物具有低毒性(LD50 = 129 mg/kg),可作为开发新型抗真菌药物的主要候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


