An alkali-free synthesis strategy for high-yield unsymmetrical indocyanine dyes
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
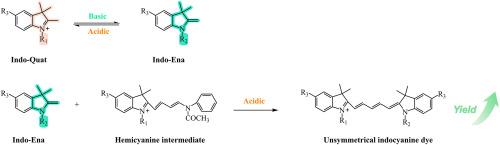
Unsymmetrical indocyanine dyes have extremely important applications in the field of biological detection. To address the key challenges of low yield and purity during the synthesis of these compounds, a new method via a two-step process is proposed for the synthesis of a series of the unsymmetrical indocyanine dyes. In the first step, an indole with a quaternary ammonium structure is reacted with a condensing agent in acetic anhydride to synthesize the hemicyanine intermediate. In the second step, an indole with an enamine structure is reacted with the hemicyanine intermediate to synthesize the unsymmetrical indocyanine dye with no additional base catalyst. This effectively inhibits the reverse reaction of the hemicyanine intermediates to indole with a quaternary ammonium structure in basic conditions, thereby suppressing the formation of indocyanine byproducts that are difficult to separate. As a result, the yields of the unsymmetrical indocyanine dyes have been greatly improved. The higher nucleophilicity of indole containing an enamine structure than indole with a quaternary ammonium structure has been reasonable explained by DFT calculations of Frontier Molecular Orbitals and electrostatic surface potentials. This study provided a new strategy for synthesizing high-yield unsymmetrical indocyanine dyes.
高产不对称吲哚菁染料的无碱合成策略
不对称吲哚菁染料在生物检测领域有着极其重要的应用。为解决不对称吲哚菁染料在合成过程中收率低、纯度低的关键问题,提出了一种两步法合成一系列不对称吲哚菁染料的新方法。在第一步中,将具有季铵结构的吲哚与凝聚剂在乙酸酐中反应以合成半苯胺中间体。在第二步中,具有烯胺结构的吲哚与半苯胺中间体反应合成不对称的吲哚苯胺染料,无需附加碱催化剂。这有效地抑制了半氨基苯胺中间体在碱性条件下与季铵结构吲哚的逆反应,从而抑制了难以分离的半氨基苯胺副产物的形成。结果表明,不对称吲哚菁染料的产率大大提高。含烯胺结构的吲哚的亲核性高于季铵结构的吲哚,这一现象通过前沿分子轨道和静电表面势的DFT计算得到了合理的解释。本研究为高产不对称吲哚菁染料的合成提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


