Iodine catalysed synthesis of 4′H-dispiro[indoline-3,2′-pyrrole-3′,3″-indoline]-2,2″-dione through C–C bond formation
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Spirooxindoles have become a privileged skeleton due to their wide and promising activities in pharmaceutical field. This work highlights metal-free synthetic approach for the construction of 4′H-dispiro[indoline-3,2′-pyrrole-3′,3″-indoline]-2,2″-dione by iodine catalyzed C–C bond formation reaction. This straight-forward benign synthetic pathway results in the formation of different spirooxindoles which can be used to synthesize various naturally occurring alkaloids and drugs. The reaction involves in-situ generation of imine by reaction of easily available isatin and (+)-(R)-α-phenyl/naphthyl ethanamine followed by C–C bond formation and further cyclization to construct a well-designed spirooxindole.
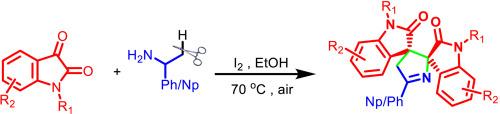
碘通过C-C键催化合成4 ' h -吡咯[吲哚-3,2 ' -吡咯-3 ',3″-吲哚]-2,2″-二酮
螺旋菌吲哚因其在制药领域的广泛应用而成为一种特殊的骨架。本工作重点介绍了用碘催化C-C键形成反应构建4'H-dispiro[吲哚-3,2 ' -吡咯-3 ',3″-吲哚]-2,2″-二酮的无金属合成方法。这种直接的良性合成途径导致形成不同的螺菌吲哚,可用于合成各种天然存在的生物碱和药物。该反应是由易获得的isatin和(+)-(R)-α-苯基/萘乙胺在原位生成亚胺,然后形成C-C键并进一步环化以构建设计良好的螺肟哚。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


