Controlling the cold-set gelation of Bovine Serum Albumin protein using alcohol and ionic surfactant
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
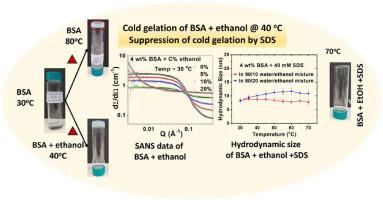
Heating of globular protein solutions usually leads to protein denaturation and subsequent gelation at high temperatures. Under “cold gelation”, protein forms a gel at a much lower temperature than its original gelation temperature (TG), which can be achieved by modifying various physicochemical conditions such as the pH of the solution, the presence of salts, etc. In this study, we investigated the cold gelation of Bovine Serum Albumin (BSA) protein induced by ethanol and controlled by ionic surfactant, using small-angle neutron scattering (SANS), dynamic light scattering (DLS), and rheology The results show that the TG of the protein with ethanol is systematically decreased as compared to the that of pure BSA solutions (∼80 °C), reaching ∼60 °C at 10 wt% ethanol, ∼55 °C at 20 wt% and finally as low as ∼38 °C in presence of 30 wt% ethanol in the solution. Rheological measurements demonstrate a significant strengthening of the gel network, with the enhancement in storage modulus (G′) from ∼20 Pa at 0 wt% to ∼250 Pa at 30 wt% ethanol. Structural characterization reveals an increase in fractal dimension with rising ethanol content, indicating denser and more branched gel networks. Interestingly, the addition of the anionic surfactant sodium dodecyl sulfate (SDS) inhibits the alcohol-assisted cold gelation of BSA protein, depending upon the relative amount of ethanol and SDS in solution. The results are explained based on the interplay of interactions in the protein, manipulated by the presence of alcohol, elevated temperatures, and ionic surfactant. Our study highlights the tunability of gelation pathways and offers useful inputs for controlled protein gelation in biomaterial and food industry.
用酒精和离子表面活性剂控制牛血清白蛋白冷凝
加热球状蛋白质溶液通常会导致蛋白质在高温下变性和随后的凝胶化。在“冷凝胶”下,蛋白质在比其原始凝胶温度(TG)低得多的温度下形成凝胶,这可以通过改变各种物理化学条件(如溶液的pH值、盐的存在等)来实现。在本研究中,我们利用小角中子散射(SANS)、动态光散射(DLS)和流变学方法,研究了离子表面活性剂控制下乙醇对牛血清白蛋白(BSA)蛋白的冷凝胶作用。结果表明,与纯BSA溶液(~ 80°C)相比,乙醇对蛋白质的热重(TG)有系统的降低,在10 wt%乙醇条件下达到~ 60°C;在20% wt%时温度为~ 55°C,最后在溶液中存在30% wt%乙醇时温度低至~ 38°C。流变学测量表明,凝胶网络显著增强,存储模量(G′)从0 wt%时的~ 20 Pa增加到30 wt%乙醇时的~ 250 Pa。结构表征表明,随着乙醇含量的增加,分形维数增加,表明凝胶网络密度更大,分支更多。有趣的是,阴离子表面活性剂十二烷基硫酸钠(SDS)的加入抑制了乙醇辅助的牛血清白蛋白冷凝胶,这取决于溶液中乙醇和SDS的相对量。结果是基于蛋白质中相互作用的相互作用,通过酒精、高温和离子表面活性剂的存在来操纵。我们的研究强调了凝胶途径的可调性,并为生物材料和食品工业中的受控蛋白质凝胶提供了有用的输入。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


