Modulation mechanism of starch gelatinization and gluten network restructuring in wheat flour during atmospheric steam treatment
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
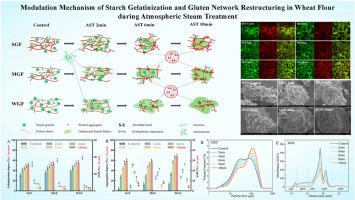
This study elucidates the competitive molecular interplay between starch gelatinization and protein aggregation in wheat flours of varying gluten strengths (strong, medium, weak) during atmospheric steam treatment (AST). Through integrated microstructural, crystallographic, spectroscopic, thermal, chromatographic, and electrophoretic analysis, we demonstrate a phase-dependent shift in dominance between starch gelatinization and protein aggregation. During initial AST (0–2 min), rapid starch hydration drives partial gelatinization, disrupting crystalline domains, reducing short-range molecular order, and promoting amylose-lipid complex formation. With extended treatment (4–10 min), dominance shifted to protein aggregation, where heat-induced conformational unfolding triggered α-helix to β-sheet transitions, exposing buried sulfhydryl groups and hydrophobic residues. These changes facilitated irreversible protein network formation via disulfide cross-linking and hydrophobic associations. Concurrently, protein aggregates adhered to starch granule surfaces, forming physical barriers that restricted water penetration and suppressed further gelatinization. Gluten strength critically modulated these competitive interactions. During initial AST (2 min), gelatinization degrees reached 30.03 %, 35.02 %, and 37.86 % for strong gluten flour (SGF), medium gluten flour (MGF), and weak gluten flour (WGF), reaching 50.83 %, 55.16 %, and 58.14 % after 10 min treatment. Following AST, SGF formed dense, cross-linked protein matrices that tightly encapsulated partially gelatinized starch fragments. In contrast, WGF formed extensive starch gel phases with relatively sparse protein aggregates, resulting in a more open network. MGF displayed a balanced structure with interpenetrating starch and protein networks reflecting intermediate structural characteristics. These findings elucidate the molecular mechanisms governing starch-protein interplay during AST, providing mechanistic foundation for process optimization and targeted wheat-based products development with desirable functional properties.
常压蒸汽处理对小麦粉淀粉糊化和面筋网络重构的调节机理
本研究阐明了常压蒸汽处理(AST)过程中不同面筋强度(强、中、弱)的小麦粉中淀粉糊化和蛋白质聚集之间的竞争性分子相互作用。通过综合微观结构、晶体学、光谱、热、色谱和电泳分析,我们证明了淀粉糊化和蛋白质聚集之间存在相依赖的优势转移。在最初的AST(0-2分钟)中,快速的淀粉水化驱动部分糊化,破坏晶体结构域,降低短程分子秩序,促进直链淀粉-脂质复合物的形成。随着处理时间延长(4-10分钟),优势转移到蛋白质聚集,热诱导的构象展开触发α-螺旋到β-薄片的转变,暴露出埋藏的巯基和疏水残基。这些变化通过二硫交联和疏水结合促进了不可逆蛋白质网络的形成。同时,蛋白质聚集体粘附在淀粉颗粒表面,形成物理屏障,限制水的渗透,抑制进一步的糊化。谷蛋白强度对这些竞争性相互作用起关键调节作用。在AST初期(2 min),强筋面粉(SGF)、中筋面粉(MGF)和弱筋面粉(WGF)的糊化度分别为30.03%、35.02%和37.86%,处理10 min后分别为50.83%、55.16%和58.14%。继AST之后,SGF形成致密的交联蛋白基质,紧密包裹部分糊化的淀粉片段。相比之下,WGF形成了广泛的淀粉凝胶相,蛋白质聚集体相对稀疏,导致网络更加开放。MGF结构平衡,淀粉和蛋白质网络相互渗透,反映了中间结构特征。这些发现阐明了AST过程中淀粉-蛋白相互作用的分子机制,为工艺优化和具有理想功能特性的靶向小麦产品开发提供了机制基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


