Exploring new strategies for improved aluminium-ion batteries through dual-anion modeling
IF 11
1区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
Abstract
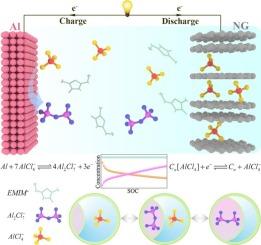
Aluminium-ion batteries (AIBs) offer numerous advantages, including high capacity, safety, and environmental sustainability, positioning them as a promising technological solution for mobile power and energy storage applications. Nevertheless, the effect of excess electrolytes reduces energy density, and the thermal instability of large-capacity batteries represents a major obstacle to large-scale applications. The energy storage mechanism of AIBs involves the reversible deposition/dissolution of metallic aluminium (Al) at the anode within a chloroaluminate ionic liquid electrolyte, coupled with the intercalation/deintercalation of AlCl4− at the graphite cathode. These processes lead to dynamic changes in the concentrations of two anions (AlCl4− and Al2Cl7−), which in turn affect conductivity, diffusion coefficients, and battery polarization. This unique dual-anion property presents challenges for understanding the operating mechanisms and studying the internal states, both of which are critical factors for developing high-performance AIBs. Based on extensive geometric, kinetic and thermodynamic data, derive and calculate the behavior of the two anions. We have pioneered an AIBs electrochemical model with dual-anion characteristics which can accurately simulate the external and internal states such as battery potential with a simulation error of less than 0.38 %, temperature and optimize the mass ratio of the cathode material to electrolyte at 1.85:1. In addition, this constructed AIBs model explores a novel strategy to improve the battery management system, increase the operation efficiency and thermal management, and create a theoretical basis for further optimization of the battery design.
通过双阴离子模型探索改进铝离子电池的新策略
铝离子电池(aib)具有许多优点,包括高容量、安全性和环境可持续性,使其成为移动电源和储能应用的一种有前途的技术解决方案。然而,过量电解质的影响降低了能量密度,大容量电池的热不稳定性是大规模应用的主要障碍。AIBs的储能机制包括金属铝(Al)在氯铝酸盐离子液体电解质中的可逆沉积/溶解,以及AlCl4−在石墨阴极的嵌入/脱嵌。这些过程导致两种阴离子(AlCl4−和Al2Cl7−)浓度的动态变化,进而影响电导率、扩散系数和电池极化。这种独特的双阴离子性质为理解其运行机制和研究其内部状态带来了挑战,而这两者都是开发高性能aib的关键因素。基于大量的几何、动力学和热力学数据,推导并计算了这两种阴离子的行为。我们率先开发了具有双阴离子特性的AIBs电化学模型,该模型可以准确模拟电池电势、温度等内外状态,模拟误差小于0.38%,并优化了正极材料与电解质的质量比为1.85:1。此外,构建的AIBs模型探索了改进电池管理系统、提高运行效率和热管理的新策略,为电池设计的进一步优化奠定了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Energy
工程技术-工程:化工
CiteScore
21.20
自引率
10.70%
发文量
1830
审稿时长
41 days
期刊介绍:
Applied Energy serves as a platform for sharing innovations, research, development, and demonstrations in energy conversion, conservation, and sustainable energy systems. The journal covers topics such as optimal energy resource use, environmental pollutant mitigation, and energy process analysis. It welcomes original papers, review articles, technical notes, and letters to the editor. Authors are encouraged to submit manuscripts that bridge the gap between research, development, and implementation. The journal addresses a wide spectrum of topics, including fossil and renewable energy technologies, energy economics, and environmental impacts. Applied Energy also explores modeling and forecasting, conservation strategies, and the social and economic implications of energy policies, including climate change mitigation. It is complemented by the open-access journal Advances in Applied Energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


