Effect of additives on the asymmetric reduction of fluorinated ketones using cyanobacterium Synechocystis sp. PCC 6803
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The asymmetric reduction of fluorinated ketones (1a–c) was investigated using the wild-type cyanobacterium Synechocystis sp. PCC 6803 at 25 °C for 24 h. Under red LED illumination (660 nm, 10 μmol m−2 s−1), the photosynthetic system promoted cofactor regeneration, enhancing the formation of optically active alcohols (2a–c). The effect of substrate concentration was found to significantly influence stereoselectivity: at low concentrations, L-alcohols were predominantly formed, whereas at higher concentrations, a shift toward D-selectivity was observed, especially for substrate 1c (41 % ee (L) at 0.05 mM; 87 % ee (D) at 5 mM). Furthermore, 1a acted as a competitive inhibitor in the reduction of 1c, effectively altering the enantioselectivity. Additionally, hyperosmotic conditions induced by 0.5 M sorbitol or 0.25 M NaCl increased the yield of L-alcohols, likely by upregulating the expression of the fabG gene, which encodes an NADPH-dependent ketoreductase. These findings provide new insights into the control of stereoselectivity and reaction efficiency in photobiocatalytic reductions using whole-cell cyanobacteria.
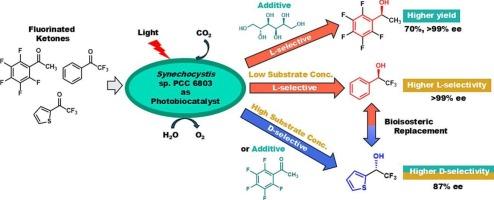
添加剂对聚胞蓝藻不对称还原氟酮的影响
利用野生型蓝细菌Synechocystis sp. PCC 6803,在25°C、24 h下研究了氟化酮(1a-c)的不对称还原。在红色LED照明(660 nm、10 μmol m−2 s−1)下,光合系统促进了辅因子的再生,促进了光学活性醇(2a-c)的形成。发现底物浓度的影响显著影响立体选择性:在低浓度下,主要形成L-醇,而在较高浓度下,观察到向d -选择性的转变,特别是底物1c (41% ee (L), 0.05 mM;87% ee (D)在5毫米)。此外,1a在1c的还原中起到竞争性抑制剂的作用,有效地改变了对映体的选择性。此外,0.5 M山梨醇或0.25 M NaCl诱导的高渗条件增加了l -醇的产量,可能是通过上调编码nadph依赖性酮还原酶的fabG基因的表达。这些发现为利用全细胞蓝藻控制光催化还原中的立体选择性和反应效率提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


