Recent advances and applications in integrating nucleic acid amplification technologies and CRISPR systems by spatial / temporal separation strategies for one-pot diagnosis
IF 12
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The integration of nucleic acid amplification techniques (NAATs) with Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) systems has enabled a new generation of molecular diagnosis that combines high sensitivity with single-nucleotide specificity. More importantly, consolidating both processes into a unified and airtight “one-pot” format without “non-closed” reagent transfer steps holds substantial promise for point-of-care testing (POCT) in resource-limited settings, particularly in clinical situations requiring rapid responses, such as during pandemics. This approach offers streamlined workflows, faster turnaround times, and reduced risk of contamination. However, the intrinsic biochemical incompatibility between amplification enzymes and CRISPR nucleases, as the former typically generate more nucleic acids while the latter consumes them to produce a signal, particularly due to competing reaction kinetics, poses a major challenge for one-pot assay development. In recent years, significant progress has been made in addressing this issue through a range of biochemical and engineering strategies. This review systematically categorizes these strategies based on whether NAATs and CRISPR systems are fully separated at the onset of the reaction, classifying them into spatial separation and temporal separation approaches. We highlight key representative studies within each category and evaluate their underlying mechanisms, performance trade-offs, and practical limitations. Furthermore, we discuss the remaining challenges that hinder clinical translation and provide insights into future directions, such as integration with upstream sample preparation and downstream signal readout modules. We hope this review not only provides a coherent roadmap for the rational design of one-pot platforms but also inspires broader innovation at the interface of molecular engineering in real-world healthcare applications.
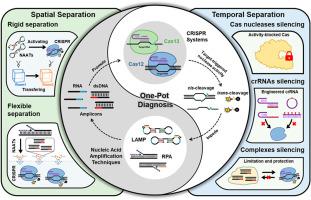
基于时空分离策略整合核酸扩增技术与CRISPR系统的“一锅诊断”研究进展及应用
核酸扩增技术(NAATs)与聚集规则间隔短回文重复序列(CRISPR)系统的整合,使新一代分子诊断具有高灵敏度和单核苷酸特异性。更重要的是,将这两个过程整合成一个统一的、密封的“一个锅”格式,而不需要“非封闭”的试剂转移步骤,这为在资源有限的环境下,特别是在需要快速反应的临床情况下(如在大流行期间)进行即时检测(POCT)带来了巨大的希望。这种方法提供了简化的工作流程,更快的周转时间,并降低了污染风险。然而,扩增酶和CRISPR核酸酶之间固有的生化不相容性,因为前者通常产生更多的核酸,而后者消耗它们来产生信号,特别是由于反应动力学的竞争,这给一锅分析的发展带来了重大挑战。近年来,通过一系列生物化学和工程策略解决这一问题取得了重大进展。本文根据naat和CRISPR系统在反应开始时是否完全分离,系统地对这些策略进行了分类,并将其分为空间分离和时间分离两种方法。我们重点介绍了每个类别中的关键代表性研究,并评估了它们的潜在机制、性能权衡和实际限制。此外,我们还讨论了阻碍临床翻译的其他挑战,并提供了对未来方向的见解,例如与上游样品制备和下游信号读出模块的集成。我们希望这篇综述不仅为一锅平台的合理设计提供了一个连贯的路线图,而且还能激发现实世界医疗保健应用中分子工程界面的更广泛创新。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Trends in Analytical Chemistry
化学-分析化学
CiteScore
20.00
自引率
4.60%
发文量
257
审稿时长
3.4 months
期刊介绍:
TrAC publishes succinct and critical overviews of recent advancements in analytical chemistry, designed to assist analytical chemists and other users of analytical techniques. These reviews offer excellent, up-to-date, and timely coverage of various topics within analytical chemistry. Encompassing areas such as analytical instrumentation, biomedical analysis, biomolecular analysis, biosensors, chemical analysis, chemometrics, clinical chemistry, drug discovery, environmental analysis and monitoring, food analysis, forensic science, laboratory automation, materials science, metabolomics, pesticide-residue analysis, pharmaceutical analysis, proteomics, surface science, and water analysis and monitoring, these critical reviews provide comprehensive insights for practitioners in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


