Resource utilization of copper slag: The enhanced sulfidization mechanism of Pb2+/NH4+ system on copper oxide in copper slag and its impact on flotation performance
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The hydrophilic nature of the copper oxide phase in copper slag (CS) often results in low recovery using traditional sulfidization flotation methods. This study explored the influence of Pb2+/NH4+ on the flotation efficiency and its activation mechanism during the sulfidization flotation of copper oxide. Analytical techniques including microcalorimetry, scanning electron microscopy–energy dispersive X-ray spectroscopy (SEM–EDS), time–of–flight secondary ion mass spectrometry (ToF–SIMS), and X–ray photoelectron spectroscopy (XPS) revealed that the treatment of Pb2+/NH4+ enhanced the reaction between sodium sulfide and the copper oxide surface. This modification facilitated the formation of sulfide compounds such as lead–sulfur (Pb–S) and copper–sulfur (Cu–S). The formation of these sulfide products enabled the surface of copper oxide to exhibit more active sites for collector adsorption, thereby enhancing its hydrophobicity and flotation performance. Further confirmation from Fourier transform infrared spectroscopy (FT–IR) and ToF–SIMS analysis revealed that Pb2+/NH4+ significantly enhanced the adsorption of collectors on the copper oxide surface, thereby enhancing its hydrophobicity. Micro–flotation experiments demonstrated that the incorporation of Pb2+\NH4+ enhanced copper oxide recovery from 40 % to 91 %, which validated their effective activation role in copper oxide flotation.
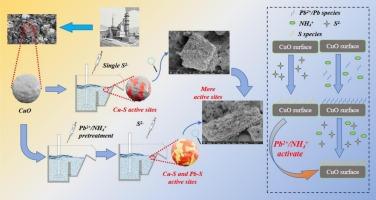
铜渣资源利用:Pb2+/NH4+体系对铜渣中氧化铜的强化硫化机理及其对浮选性能的影响
由于铜渣中氧化铜相的亲水性,采用传统的硫化浮选方法往往回收率较低。研究了氧化铜硫化浮选中Pb2+/NH4+对浮选效率的影响及其活化机理。微量热法、扫描电镜- x射线能谱(SEM-EDS)、飞行时间二次离子质谱(ToF-SIMS)和x射线光电子能谱(XPS)等分析技术表明,Pb2+/NH4+处理增强了硫化钠与氧化铜表面的反应。这种改性促进了硫铅(Pb-S)和铜硫(Cu-S)等硫化物的形成。这些硫化产物的形成使氧化铜表面呈现出更多的捕收剂吸附活性位点,从而增强了其疏水性和浮选性能。傅里叶变换红外光谱(FT-IR)和ToF-SIMS分析进一步证实,Pb2+/NH4+显著增强了捕集剂在氧化铜表面的吸附,从而增强了其疏水性。微浮选实验表明,掺入Pb2+\NH4+可使氧化铜回收率从40%提高到91%,验证了其在氧化铜浮选中的有效活化作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


