Catalytic baeyer-villiger oxidation over N-doped carbon with aliphatic aldehyde: Mechanism, kinetic modeling, life cycle assessment, and techno-economic analysis
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
ε-Caprolactone (ε-CL) is a key organic chemical intermediate and monomer for polycaprolactone (PCL). Developing a safe and cost-effective process for its efficient synthesis remains challenging. Here, a Baeyer-Villiger oxidation of cyclohexanone (Cy=O) using propionaldehyde (PRA) as a co-oxidant and N-doped carbon loaded carbon nanotubes (NDC@CNTs) as catalysts was conducted with a feature of the radical free reaction. The process achieves 40.14 % Cy=O conversion, 93.41 % ε-CL selectivity, and 0.36 aldehyde efficiency (AE). Good values of TON, STY and E-factor for the NDC@CNTs-catalyzed system showed the good scalability potential. The structure–activity relationship investigation showed that as the nitrogen content in NDC@CNTs increased, reaction activity rose and then declined. The ratio of pyridinic N to graphitic N (NPy/NG) was the key factor affecting activity. Mechanistic research revealed that NDC@CNTs boost overall reaction activity by accelerating PRA oxidation to peroxypropionic acid, which was crucial for the reaction. Kinetic studies determined the reaction orders and rate constants for Cy=O oxidation and PRA self-oxidation, establishing a predictable kinetic model through semi-batch reactions. LCA analysis reveals that this reaction system exerts a comparatively low pressure on global environmental reference systems. Moreover, the entire reaction process was meticulously designed and simulated using Aspen Plus software with a 98.77 % likelihood of positive unit profit (622.22 ∼ 2909.06 USD/t), highlighting the good economic value.
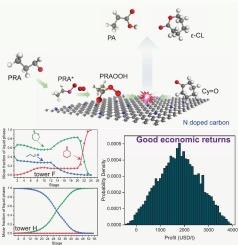
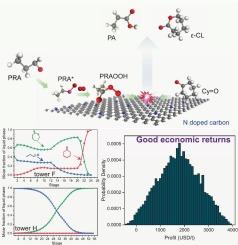
n掺杂碳与脂肪醛催化baeyer-villiger氧化:机理、动力学建模、生命周期评估和技术经济分析
ε-己内酯(ε-CL)是聚己内酯(PCL)的重要有机中间体和单体。开发一种安全且具有成本效益的高效合成工艺仍然具有挑战性。本研究以丙醛(PRA)为共氧化剂,氮掺杂碳纳米管(NDC@CNTs)为催化剂,进行了环己酮(Cy=O)的Baeyer-Villiger氧化反应。该工艺的Cy=O转化率为40.14 %,ε-CL选择性为93.41 %,醛效率(AE)为0.36。NDC@CNTs-catalyzed系统的TON、STY和E-factor值均较好,显示出良好的可扩展性。构效关系研究表明,随着NDC@CNTs中氮含量的增加,反应活性先升后降。吡啶氮与石墨氮的比值(NPy/NG)是影响活性的关键因素。机制研究表明NDC@CNTs通过加速PRA氧化成过氧丙酸来提高整个反应活性,这对反应至关重要。动力学研究确定了Cy=O氧化和PRA自氧化的反应级数和速率常数,通过半批反应建立了可预测的动力学模型。LCA分析表明,该反应体系对全球环境参考系统的压力相对较低。此外,整个反应过程经过精心设计,并使用Aspen Plus软件进行模拟,其正单位利润可能性为98.77 %(622.22 ~ 2909.06美元/吨),突出了良好的经济价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


