Self-driven aldol condensation enabling high-purity Li2CO3 recovery from spent lithium metal anodes
IF 35.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Rechargeable lithium (Li)-metal batteries with high energy densities are approaching large-scale commercialization but face challenges in recycling due to the high reactivity of spent Li-metal anodes (S-LMAs). Here, we propose a recovery strategy using commercial acetone containing <1.00 wt % water. Sub-percent water first reacts with S-LMA to form LiOH, which consumes Li dendrites and mitigates safety risks. Then, LiOH catalyzes acetone aldol condensation to low-concentration diacetone alcohol (DAA, <5 mol %), which further reacts with S-LMA at acceptable rates and simultaneously drives the DAA production, thus achieving complete conversion of S-LMA. This approach yields high-purity Li2CO3 (99.79 wt %), surpassing the battery-grade standard (99.50 wt %). The recovered Li2CO3 enables the synthesis of LiNi0.6Mn0.2Co0.2O2 cathodes with electrochemical performance comparable to those from commercial Li2CO3. Combining safety, scalability, and economic viability, this method provides a practical route for recycling rechargeable Li-metal batteries and offers insights for extending to other alkali-metal-based batteries.
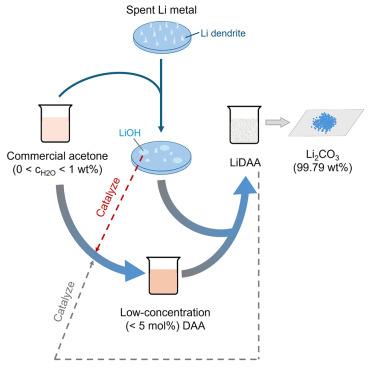
从废锂金属阳极中回收高纯度Li2CO3的自驱动醛醇缩合
具有高能量密度的可充电锂(Li)金属电池正接近大规模商业化,但由于废锂金属阳极(S-LMAs)的高反应性,在回收方面面临挑战。在这里,我们提出了一种回收策略,使用含有<;1.00 wt %水的商业丙酮。低于百分之一的水首先与S-LMA反应形成LiOH, LiOH消耗了Li枝晶,降低了安全风险。然后,LiOH催化丙酮醛缩合生成低浓度的二丙酮醇(DAA, < 5mol %),再与S-LMA以可接受的速率反应,同时驱动DAA的生成,从而实现S-LMA的完全转化。这种方法可以产生高纯度的Li2CO3 (99.79 wt %),超过电池级标准(99.50 wt %)。回收的Li2CO3可以合成电化学性能与商用Li2CO3相当的LiNi0.6Mn0.2Co0.2O2阴极。该方法结合了安全性、可扩展性和经济可行性,为可充电锂金属电池的回收提供了一条实用的途径,并为扩展到其他碱金属电池提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Joule
Energy-General Energy
CiteScore
53.10
自引率
2.00%
发文量
198
期刊介绍:
Joule is a sister journal to Cell that focuses on research, analysis, and ideas related to sustainable energy. It aims to address the global challenge of the need for more sustainable energy solutions. Joule is a forward-looking journal that bridges disciplines and scales of energy research. It connects researchers and analysts working on scientific, technical, economic, policy, and social challenges related to sustainable energy. The journal covers a wide range of energy research, from fundamental laboratory studies on energy conversion and storage to global-level analysis. Joule aims to highlight and amplify the implications, challenges, and opportunities of novel energy research for different groups in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


