p-Alkoxy-Substituted Anisomycins with Potent Anti-Trypanosomiasis Activity and Expanded Modes of Action
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
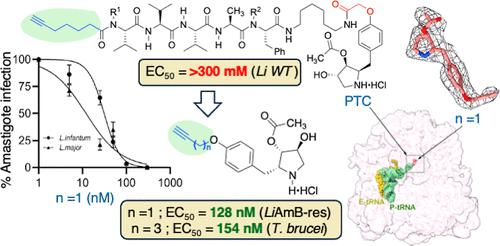
Neglected tropical diseases caused by trypanosomatid parasites present a major public healthcare issue, partly due to emerging resistance. Attachment of ω-alkynyl chains characteristic of the lipid tails of antiparasitic peptides to the p-position of anisomycin gave ethers exhibiting potent activity, rivalling that of the parent ribosomal inhibitor, especially against resistant Leishmania strains. Single-particle cryoelectron microscopy analysis revealed that O-propargyl anisomycin binds to the highly conserved peptidyl transferase center of the ribosome similar to the parent inhibitor. Thermal proteomic profiling and gene ontology analysis demonstrated that O-propargyl anisomycin exhibited a broader mode of action, including activity against glycosome-associated proteins. Alkynyl substituents improved antiparasitic activity against resistant strains, likely by enlarging the mode of action, offering a novel path toward therapy against trypanosomatid infections.
具有有效抗锥虫病活性和扩展作用方式的对烷氧基取代的异菌素
由锥虫虫寄生虫引起的被忽视的热带病是一个主要的公共卫生问题,部分原因是出现了耐药性。抗寄生虫肽脂质尾部的ω-炔基链附着在大霉素的p位上,产生的醚具有强大的活性,与母体核糖体抑制剂相媲美,特别是对耐药利什曼原虫菌株。单粒子冷冻电镜分析显示,o -丙炔大霉素与核糖体高度保守的肽基转移酶中心结合,与母体抑制剂相似。热蛋白质组学分析和基因本体分析表明,o -丙炔大霉素具有更广泛的作用模式,包括对糖体相关蛋白的活性。炔基取代基提高了对耐药菌株的抗寄生活性,可能是通过扩大作用模式,为治疗锥虫感染提供了一条新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


