Cyclodextrin: A spectroscopic and in-silico investigation as a size-dependent cytoplasmic protein stabilizer
IF 4.6
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2025-09-15
DOI:10.1016/j.saa.2025.126948
引用次数: 0
Abstract
Understanding the effect of the surrounding environment of the cytoplasm on protein stability gives insight into developing successful drug-delivery vehicles and biopharmaceutical formulations. Here we investigate the interaction of a small, monomeric cytoplasmic protein, Cellular retinoic acid binding protein I (CRABPI), with natural polysaccharides, cyclodextrins (α-, β-, and γ-CD) of different sizes. The characteristic toroidal shape with a hydrophobic cavity allows interaction with the protein surface through host-guest interactions. Using different multi-dimensional approaches, spectroscopic analyses, and in silico studies gives an impactful observation on structural and conformational stability. The output highlights that γ-cyclodextrin has the highest binding affinity and stabilization effect. Enhancement of fluorescence intensity, lifetime, and secondary contents of protein with a decrease in structural fluctuations in in silico study strongly supports the outcome. β-CD shows moderate stabilizing behaviour, while α-CD engages primarily in superficial hydrogen bonding with minimal impact on protein conformation. It is significant to highlight that the large cavity size of γ-CD encapsulates the surface amino acids of CRABP I, supporting localized structural rigidification without disturbing the global fold of the protein. These results establish a clear structure–function relationship among the CDs and highlight their potential as targeted molecular stabilizers. Our results underscore the importance of γ-cyclodextrin as a promising molecular chaperone in protein delivery and stabilization strategies, thereby extending cyclodextrin applications beyond drug encapsulation towards potential roles in structural stabilization of cytoplasmic proteins (like CRABPI).
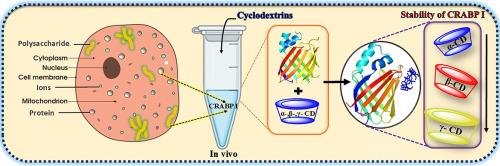
环糊精:作为尺寸依赖性细胞质蛋白稳定剂的光谱和硅研究。
了解细胞质周围环境对蛋白质稳定性的影响,有助于开发成功的药物递送载体和生物制药配方。在这里,我们研究了一个小的,单体的细胞质蛋白,细胞维甲酸结合蛋白I (CRABPI),与天然多糖,环糊精(α-, β-和γ-CD)的相互作用。具有疏水腔的特征环形允许通过主-客体相互作用与蛋白质表面相互作用。使用不同的多维方法,光谱分析和硅研究给出了对结构和构象稳定性的有效观察。结果表明,γ-环糊精具有最高的结合亲和力和稳定作用。荧光强度、寿命和蛋白质二级含量的增强与结构波动的减少在硅研究中有力地支持了这一结果。β-CD表现出适度的稳定行为,而α-CD主要参与表面氢键,对蛋白质构象的影响最小。值得注意的是,γ-CD的大空腔尺寸封装了CRABP I的表面氨基酸,支持局部结构刚性而不干扰蛋白质的全局折叠。这些结果建立了CDs之间清晰的结构功能关系,并突出了它们作为靶向分子稳定剂的潜力。我们的研究结果强调了γ-环糊精作为一种有前途的分子伴侣在蛋白质递送和稳定策略中的重要性,从而将环糊精的应用范围从药物包封扩展到细胞质蛋白(如CRABPI)的结构稳定方面的潜在作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


