Arachidonic acid regulates hormonal crosstalk to promote molting success and mediate a life-history trade-off in a wolf spider
IF 2.3
2区 农林科学
Q1 ENTOMOLOGY
引用次数: 0
Abstract
The biochemical composition of prey is a critical factor shaping the life-history strategies of obligate predators, yet the molecular mechanisms through which specific nutrients regulate complex developmental processes remain largely unknown. Using the wolf spider Pardosa pseudoannulata as a model, where arachidonic acid (ARA) is indispensable for preventing molting death, we integrated life-history analysis and RNA sequencing to elucidate its regulatory role. We discovered a significant life-history trade-off: while essential for survival, dietary ARA significantly prolonged early instar duration. Transcriptomic analysis revealed that ARA orchestrates a two-phase developmental strategy to resolve this conflict. The “preparation” phase, marked by the upregulation of juvenile hormone synthesis (JHAMT), corresponds to the developmental delay and facilitates resource accumulation. This is followed by an “execution” phase, where ARA triggers the entire ecdysone cascade, from biosynthesis (e.g., Spook, Shade) and signaling (USP) to catabolism (CYP18A1). This hormonal activation was coupled with the substantial upregulation of downstream effector genes, including dozens of cuticular proteins. Our findings indicate that ARA acts as a key signaling molecule that coordinates the hormonal crosstalk between developmental timing and molting. This study provides a comprehensive molecular model for the nutritional regulation of arthropod development, linking nutrition, physiology, and survival.
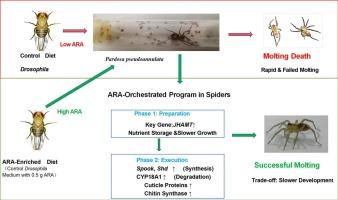
花生四烯酸调节激素串扰促进狼蛛成功换羽和调节生活史权衡。
猎物的生物化学组成是决定专性捕食者生活史策略的关键因素,但特定营养物质调节复杂发育过程的分子机制在很大程度上仍然未知。以狼蛛为研究对象,结合生活史分析和RNA测序,研究了花生四烯酸(ARA)在预防脱毛死亡中不可或缺的作用。我们发现了一个重要的生活史权衡:虽然饮食ARA对生存至关重要,但它显著延长了早期持续时间。转录组学分析显示,ARA协调了两个阶段的发展策略来解决这种冲突。“准备”期以幼体激素合成(JHAMT)上调为标志,与发育迟缓相对应,有利于资源积累。接下来是“执行”阶段,ARA触发整个蜕皮激素级联反应,从生物合成(例如,Spook, Shade)和信号传导(USP)到分解代谢(CYP18A1)。这种激素激活伴随着下游效应基因的大量上调,包括数十种角质层蛋白。我们的研究结果表明,ARA作为一个关键的信号分子,协调发育时间和蜕皮之间的激素串扰。本研究为节肢动物发育的营养调控提供了一个综合的分子模型,将营养、生理和生存联系起来。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of insect physiology
生物-昆虫学
CiteScore
4.50
自引率
4.50%
发文量
77
审稿时长
57 days
期刊介绍:
All aspects of insect physiology are published in this journal which will also accept papers on the physiology of other arthropods, if the referees consider the work to be of general interest. The coverage includes endocrinology (in relation to moulting, reproduction and metabolism), pheromones, neurobiology (cellular, integrative and developmental), physiological pharmacology, nutrition (food selection, digestion and absorption), homeostasis, excretion, reproduction and behaviour. Papers covering functional genomics and molecular approaches to physiological problems will also be included. Communications on structure and applied entomology can be published if the subject matter has an explicit bearing on the physiology of arthropods. Review articles and novel method papers are also welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


