Gestational or lactational exposure to L-mimosine in rats: maternal toxicity, fetal growth retardation, and compromised skeletal development
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
L-mimosine, a compound found in legumes of the Leucaena genus, is known for its ability to disrupt the cell cycle and its toxic properties. Its effects during critical periods such as gestation and lactation remain poorly understood. This study aimed to evaluate the potential toxic effects of L-mimosine in rats exposed during these periods, as well as its transfer through biological fluids, such as milk, to offspring. Oral doses of 140, 240, or 340 mg/kg of maternal body weight were administered, and a control group (CO), which received only the vehicle, was included. During gestation, dams received L-mimosine from gestational days 6–21, whereas exposure through lactation occurs from postnatal days (PNDs) 1–21. Maternal and offspring body weight gain (BWG), maternal food consumption, and clinical signs were monitored, and offspring physical and reflex development was also assessed. Milk and maternal blood samples were collected for L-mimosine quantification, and the phytotoxin was detected in both biological fluids. Following in utero exposure, marked toxic effects were observed, especially at the 240 mg/kg dose. In dams, significant reductions (P < 0.05) in total BWG and food intake, alopecia, decreased relative right kidney weight, increased relative thymus weight, and splenic lymphoid hyperplasia were recorded. The offspring presented significant reductions (P < 0.05) in litter weight at birth and at PND 42, an increased number of stillbirths, delayed incisor eruption, and physical malformations. Histopathological analyses revealed mild to moderate alterations in the kidneys and spleen. In contrast, lactational exposure resulted in no noteworthy maternal or offspring effects, despite confirmed L-mimosine excretion in milk. These findings underscore the pronounced vulnerability of the developing organism to L-mimosine when exposure occurs in utero, emphasizing the importance of gestational periods as critical windows for toxicological risk.
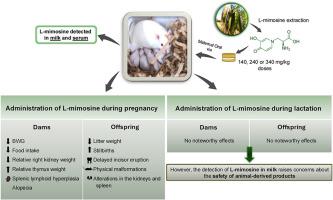
大鼠妊娠期或哺乳期暴露于左旋蜜胺:母体毒性、胎儿生长迟缓和骨骼发育受损。
l -含羞草胺是一种在Leucaena属豆科植物中发现的化合物,以其破坏细胞周期的能力和毒性而闻名。它在妊娠期和哺乳期等关键时期的影响仍然知之甚少。本研究旨在评估在这些时期暴露于l -氨基糖胺的大鼠的潜在毒性作用,以及其通过生物液体(如牛奶)向后代的转移。给予140、240或340 mg/kg母亲体重的口服剂量,并包括只接受载体的对照组(CO)。在妊娠期间,母鼠从妊娠第6-21天开始接受l -蜜胺,而通过哺乳期接触则发生在出生后第1-21天。监测母体和子代体重增加(BWG)、母体食物消耗和临床症状,并评估子代的身体和反射发育。采集乳汁和母亲血液样本进行l -氨基葡萄糖定量测定,并在两种生物体液中检测植物毒素。在子宫内暴露后,观察到明显的毒性作用,特别是在240 mg/kg剂量下。在水坝中,显著减少(P
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


