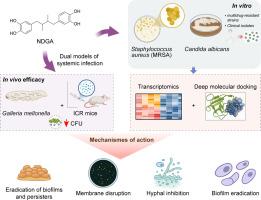
Nordihydroguaiaretic acid (NDGA) exhibits potent anti-biofilm and antimicrobial activity against methicillin-resistant Staphylococcus aureus and Candida albicans
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Introduction
The global rise of antimicrobial resistance (AMR), particularly among biofilm-forming bacteria and fungi, has created an urgent need for novel therapeutics. Nordihydroguaiaretic acid (NDGA), a plant-derived polyphenolic lignan, has demonstrated promising biological activities, yet its potential as a dual-action antimicrobial agent remains underexplored.
Objectives
This study aimed to evaluate the antimicrobial and anti-biofilm activities of NDGA against methicillin-resistant Staphylococcus aureus (MRSA) and Candida albicans, elucidate its mechanism of action, and assess its in vivo efficacy and biosafety.
Materials and Methods
NDGA’s antimicrobial activities were assessed through MIC, time-kill, and biofilm disruption assays against standard and clinical isolates. Transcriptomic profiling and deep-learning–guided molecular docking were used to identify key microbial targets and regulatory pathways. In vivo efficacy was validated in Galleria mellonella and murine systemic infection models. Cytotoxicity, hemolysis, and acute toxicity assays were conducted to evaluate biosafety.
Results
NDGA exhibited potent antimicrobial activity, with MIC values of 32 μg/ml for S. aureus USA300 and 64 μg/ml for C. albicans SC5314. It effectively eradicated persister cells and disrupting mature biofilms of both MRSA and C. albicans. Transcriptomic analysis revealed that NDGA modulated multiple microbial virulence and biofilm-regulating pathways, including arginine biosynthesis in MRSA and ergosterol metabolism in C. albicans. Docking studies confirmed strong binding affinity of NDGA to critical microbial targets. In vivo, NDGA significantly improved survival rates, reduced pathogen burden, and alleviated tissue damage, showing comparable efficacy to vancomycin and fluconazole. NDGA demonstrated favorable biosafety with low cytotoxicity, minimal hemolysis, and no observable acute toxicity in mammalian models.
Conclusion
NDGA is a promising antimicrobial candidate capable of disrupting biofilms and overcoming drug resistance in both bacterial and fungal infections. Its multitargeted mode of action, coupled with in vivo efficacy and biosafety, supports further development as a next-generation anti-infective agent.
北二氢愈创木酸(NDGA)对耐甲氧西林金黄色葡萄球菌和白色念珠菌表现出有效的抗生物膜和抗菌活性。
导言:全球抗菌素耐药性(AMR)的上升,特别是在生物膜形成细菌和真菌中,已经产生了对新型治疗方法的迫切需求。北二氢愈创木脂酸(NDGA)是一种植物衍生的多酚木脂素,具有良好的生物活性,但其作为双作用抗菌药物的潜力尚未得到充分开发。目的:评价NDGA对耐甲氧西林金黄色葡萄球菌(MRSA)和白色念珠菌的抑菌活性和抗生物膜活性,阐明其作用机制,并评价其体内疗效和生物安全性。材料和方法:NDGA的抗菌活性通过MIC、时间杀伤和生物膜破坏试验对标准和临床分离株进行评估。转录组学分析和深度学习引导的分子对接用于识别关键的微生物靶点和调控途径。在mellonia Galleria和小鼠全身感染模型中验证了体内疗效。通过细胞毒性、溶血和急性毒性试验来评价生物安全性。结果:NDGA对金黄色葡萄球菌USA300的MIC值为32 μg/ml,对白色念珠菌SC5314的MIC值为64 μg/ml。它有效地根除了持久性细胞,破坏了MRSA和白色念珠菌的成熟生物膜。转录组学分析显示,NDGA调节了多种微生物毒力和生物膜调节途径,包括MRSA的精氨酸生物合成和白色念珠菌的麦角甾醇代谢。对接研究证实NDGA对关键微生物靶点具有很强的结合亲和力。在体内,NDGA显著提高了生存率,减轻了病原体负担,减轻了组织损伤,其疗效与万古霉素和氟康唑相当。NDGA在哺乳动物模型中表现出良好的生物安全性,具有低细胞毒性,最小溶血,无明显急性毒性。结论:NDGA是一种很有前途的抗微生物候选药物,能够破坏细菌和真菌感染的生物膜并克服耐药性。它的多靶点作用模式,加上体内有效性和生物安全性,支持作为下一代抗感染药物的进一步发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


