Early-life stress and maternal immune activation alter stress coping behavior and brain metabolic activity in young male rats
IF 2.5
3区 医学
Q2 BEHAVIORAL SCIENCES
引用次数: 0
Abstract
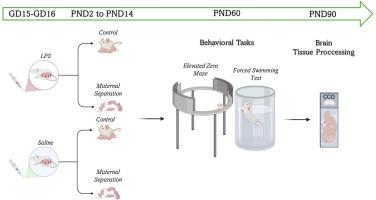
Early-life stress (ELS) and maternal immune activation (MIA) are two major environmental risk factors for the development of affective disorders. While their individual effects have been extensively studied, their combined impact on adult affective behavior and brain metabolism remains unclear. This study investigated the independent and interactive effects of MIA (LPS, bacterial lipopolysaccharide, 100 μg/kg, i.p. on gestational days 15–16) and ELS (maternal separation of pups from postnatal days 2 to 14) on affective behavior and brain oxidative metabolism in young male rats. Behavioral outcomes were assessed using the elevated zero maze and the forced swim test. Regional brain activity was quantified by cytochrome c oxidase (CCO) histochemistry in cortico-limbic and subcortical regions.
MIA significantly increased anxiety-like behavior, whereas ELS reduced locomotor activity in the elevated zero maze. A synergistic effect of both insults was observed in the forced swim test, with combined MIA and ELS animals displaying the highest immobility time. At the neurobiological level, both MIA and ELS independently increased brain CCO activity across several subcortical regions, including the amygdala, striatum, ventral hippocampus, thalamus, and substantia nigra. Neurobehavioral correlation analyses revealed distinct patterns linking behavioral outcomes with regional metabolic activity, particularly in the nucleus accumbens and bed nucleus of the stria terminalis.
These findings suggest that MIA and ELS exert synergistic effects on stress coping behavior and long-term brain oxidative metabolism. Combined exposure to gestational immune challenge and early postnatal stress may exacerbate vulnerability to developing mood disorders in adulthood by disrupting the functional development of affective- and motivation-related brain regions.
早期生活压力和母体免疫激活改变了年轻雄性大鼠的应激应对行为和脑代谢活动。
早期生活压力(ELS)和母体免疫激活(MIA)是情感性障碍发生的两个主要环境危险因素。虽然它们的个体影响已被广泛研究,但它们对成人情感行为和脑代谢的综合影响仍不清楚。本实验研究了MIA (LPS,细菌脂多糖,100 μg/kg,孕15 ~ 16天ig)和ELS(产后2 ~ 14天母仔分离)对幼龄雄性大鼠情感行为和脑氧化代谢的独立和交互作用。行为结果评估采用提升零迷宫和强迫游泳测试。采用细胞色素c氧化酶(CCO)组织化学方法定量测定皮质边缘区和皮质下区的脑活动。MIA显著增加了焦虑样行为,而ELS则减少了高零迷宫中的运动活动。在强迫游泳试验中观察到两种损伤的协同效应,MIA和ELS联合使用的动物表现出最长的静止时间。在神经生物学水平上,MIA和ELS分别增加了几个皮质下区域的大脑CCO活动,包括杏仁核、纹状体、腹侧海马、丘脑和黑质。神经行为相关分析揭示了行为结果与区域代谢活动的不同模式,特别是在伏隔核和终纹床核。这些结果表明,MIA和ELS在应激应对行为和长期脑氧化代谢方面具有协同作用。同时暴露于妊娠期免疫挑战和产后早期压力可能会破坏与情感和动机相关的大脑区域的功能发育,从而加剧成年后情绪障碍的易感性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Physiology & Behavior
医学-行为科学
CiteScore
5.70
自引率
3.40%
发文量
274
审稿时长
47 days
期刊介绍:
Physiology & Behavior is aimed at the causal physiological mechanisms of behavior and its modulation by environmental factors. The journal invites original reports in the broad area of behavioral and cognitive neuroscience, in which at least one variable is physiological and the primary emphasis and theoretical context are behavioral. The range of subjects includes behavioral neuroendocrinology, psychoneuroimmunology, learning and memory, ingestion, social behavior, and studies related to the mechanisms of psychopathology. Contemporary reviews and theoretical articles are welcomed and the Editors invite such proposals from interested authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


