Proteomic profiling of macrophages: effects of inflammatory activation and anti-inflammatory treatment with IBD therapeutics
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-18
DOI:10.1016/j.ejpb.2025.114869
引用次数: 0
Abstract
Inflammatory bowel diseases (IBD) are chronic disorders characterized by persistent immune dysregulation in the intestinal mucosa, with macrophages playing a central role in disease pathogenesis. In this study, primary human monocyte-derived macrophages (MDMs) were stimulated with lipopolysaccharide (LPS) to model innate immune activation, and subsequent proteomic changes were analyzed by mass spectrometry. The effects of three established IBD drugs, mesalazine, prednisolone and 6-mercaptopurine (6-MP), were systematically evaluated within this model. LPS stimulation resulted in activation of proteins related to pro-inflammatory pathways, including NF-κB signaling, which was reflected by increased expression of cytokine- and adhesion-related proteins such as IL1B, IL8 and ICAM1. Mesalazine treatment induced a moderate modulation of inflammatory regulators, prednisolone produced a strong suppression of pro-inflammatory and complement proteins, and 6-MP caused broad alterations in ribosomal, metabolic and apoptosis-associated proteins. These results indicate that the LPS-stimulated MDM model can reproduce essential features of macrophage activation in IBD and differentiate drug-specific proteomic signatures that are consistent with known mechanisms of action.
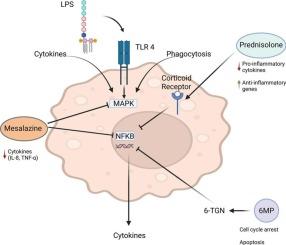
巨噬细胞的蛋白质组学分析:炎症激活和抗炎治疗对IBD治疗的影响。
炎症性肠病(IBD)是一种以肠黏膜持续免疫失调为特征的慢性疾病,巨噬细胞在疾病发病机制中起核心作用。在这项研究中,用脂多糖(LPS)刺激原代人单核细胞源性巨噬细胞(MDMs)来模拟先天免疫激活,并通过质谱分析随后的蛋白质组学变化。在该模型中系统评估了三种已建立的IBD药物,美萨拉嗪、强的松龙和6-巯基嘌呤(6-MP)的效果。LPS刺激导致促炎通路相关蛋白活化,包括NF-κ b信号,这反映在细胞因子和粘附相关蛋白如IL1B、IL8和ICAM1的表达增加。美萨拉嗪治疗诱导炎症调节因子的适度调节,强的松龙产生促炎和补体蛋白的强烈抑制,6-MP引起核糖体、代谢和凋亡相关蛋白的广泛改变。这些结果表明,lps刺激的MDM模型可以重现IBD中巨噬细胞活化的基本特征,并分化出与已知作用机制一致的药物特异性蛋白质组学特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


