MICU1 attenuates neuronal apoptosis after subarachnoid hemorrhage by inhibiting mitochondrial calcium overload and damage
IF 4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Background
Subarachnoid hemorrhage (SAH) is a severe neurological emergency associated with substantial morbidity and mortality. Research into the mechanisms underlying neuronal injury following SAH has identified early brain injury (EBI) as a critical factor influencing clinical outcomes. Among the various pathological processes involved in EBI, calcium overload remains relatively understudied yet plays a pivotal role in neuronal damage. Excessive accumulation of calcium within mitochondria can initiate apoptotic and autophagic pathways, contributing to cell death. Mitochondrial calcium uptake 1 (MICU1), a regulatory protein located on the inner mitochondrial membrane, functions to modulate mitochondrial calcium ions by inhibiting calcium influx under conditions of low intracellular calcium concentration.
Methods
Mitochondria were extracted from the cerebrospinal fluid (CSF) of patients with SAH to evaluate the extent of mitochondrial damage. In vivo and in vitro SAH models were employed to assess mitochondrial damage and dynamic changes in both mitochondrial and cytosolic calcium levels. The interaction between MICU1 and mitochondria was further examined. To investigate the functional role of MICU1, lentivirus vectors were used to upregulate MICU1 expression, while siRNA was applied to knock down its expression in Neuron-2a (N2a) cells. Following hemoglobin (Hb) stimulation, mitochondrial damage and apoptosis were systematically evaluated.
Results
Analysis of CSF from SAH patients revealed decreased MICU1 expression and aggravated mitochondrial damage. Hb stimulation of primary neurons and N2a cells led to reduced MICU1 expression and mitochondrial calcium overload, which mediated mitochondrial damage and promoted the progression of neuronal apoptosis. Following upregulation of MICU1 expression in N2a cells, the cells exhibited enhanced tolerance to Hb-induced calcium overload, resulting in a significant reduction in mitochondrial damage. This protective effect was attenuated by MICU1 siRNA treatment. Moreover, MICU1 overexpression alleviated Hb-induced apoptosis in N2a cells, whereas siRNA-mediated knockdown of MICU1 exacerbated apoptotic responses.
Conclusion
Mitochondrial calcium overload in neurons following SAH contributes to the development of EBI and neuronal damage. MICU1 exerts a neuroprotective role by mitigating mitochondrial calcium overload, thereby reducing mitochondrial damage and neuronal apoptosis.
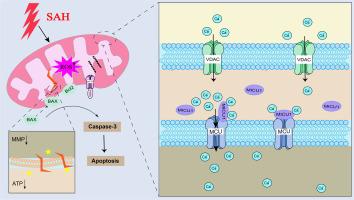
MICU1通过抑制线粒体钙超载和损伤来减轻蛛网膜下腔出血后神经元的凋亡。
背景:蛛网膜下腔出血(SAH)是一种严重的神经系统急症,具有很高的发病率和死亡率。对SAH后神经元损伤机制的研究已经确定早期脑损伤(EBI)是影响临床结果的关键因素。在EBI涉及的各种病理过程中,钙超载研究相对较少,但在神经元损伤中起关键作用。线粒体内钙的过度积累可启动凋亡和自噬途径,导致细胞死亡。线粒体钙摄取1 (MICU1)是一种位于线粒体内膜上的调节蛋白,在细胞内钙浓度低的情况下,通过抑制钙内流来调节线粒体钙离子。方法:从SAH患者脑脊液中提取线粒体,评价线粒体损伤程度。采用体内和体外SAH模型评估线粒体损伤以及线粒体和细胞质钙水平的动态变化。进一步研究了MICU1与线粒体之间的相互作用。为了研究MICU1的功能作用,我们利用慢病毒载体上调MICU1的表达,同时利用siRNA敲低其在神经元-2a (N2a)细胞中的表达。血红蛋白(Hb)刺激后,系统评估线粒体损伤和凋亡。结果:SAH患者脑脊液分析显示MICU1表达降低,线粒体损伤加重。Hb刺激原代神经元和N2a细胞导致MICU1表达降低,线粒体钙超载,介导线粒体损伤,促进神经元凋亡进程。在N2a细胞中上调MICU1表达后,细胞对hb诱导的钙超载表现出增强的耐受性,导致线粒体损伤显著减少。这种保护作用被MICU1 siRNA处理减弱。此外,MICU1过表达减轻了hb诱导的N2a细胞凋亡,而sirna介导的MICU1下调则加剧了凋亡反应。结论:SAH后神经元线粒体钙超载参与了EBI的发生和神经元损伤。MICU1通过减轻线粒体钙超载,从而减少线粒体损伤和神经元凋亡,发挥神经保护作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Cell calcium
生物-细胞生物学
CiteScore
8.70
自引率
5.00%
发文量
115
审稿时长
35 days
期刊介绍:
Cell Calcium covers the field of calcium metabolism and signalling in living systems, from aspects including inorganic chemistry, physiology, molecular biology and pathology. Topic themes include:
Roles of calcium in regulating cellular events such as apoptosis, necrosis and organelle remodelling
Influence of calcium regulation in affecting health and disease outcomes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


