Multiscale investigations of potato and wheat starch interactions with water in the absence or presence of sodium and calcium ions
IF 6.5
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2025-09-09
DOI:10.1016/j.carpta.2025.101004
引用次数: 0
Abstract
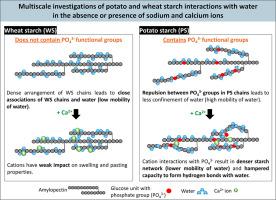
Starch interactions with water form the basis for its functional properties. Potato starch (PS) and wheat starch (WS) functionality are notably different, and largely attributed to the presence of negatively-charged phosphate monoester groups in the former and absence of these groups in the latter. In this study, sodium and calcium chloride salts were added to PS- or WS-water model systems as their cations interact with the charges of phosphate groups when present. Time domain proton nuclear magnetic resonance (TD 1H NMR) findings showed that PS exhibits higher mobility of water protons (higher T2 relaxation time) than WS. Upon cation addition, proton mobility is reduced only in PS illustrating that cation interactions with charged phosphate groups lead to stronger starch-water interactions and greater confinement of water protons in starch granules. Alongside PSs’ reduced swelling power and leaching of carbohydrates, it is suggested that cation interactions with PS hamper its capacity to form hydrogen bonds with water, thereby limiting swelling. In WS, cations can only interact with the hydroxyl groups of starch and exhibit a weaker impact on proton mobility, swelling, and pasting. These findings indicate that phosphate groups in PS considerably impact its functionality by altering its molecular-level interactions with water.
在没有或存在钠和钙离子的情况下,马铃薯和小麦淀粉与水相互作用的多尺度研究
淀粉与水的相互作用形成了其功能特性的基础。马铃薯淀粉(PS)和小麦淀粉(WS)的功能明显不同,这在很大程度上是由于前者存在带负电的磷酸单酯基团,而后者不存在这些基团。在这项研究中,钠和氯化钙盐被添加到PS-或WS-water模型系统中,因为它们的阳离子在存在时与磷酸基的电荷相互作用。时域质子核磁共振(TD 1H NMR)结果表明,PS比WS具有更高的水质子迁移率(更高的T2弛豫时间)。当阳离子加入后,质子迁移率仅在PS中降低,说明阳离子与带电磷酸基团的相互作用导致淀粉-水相互作用更强,淀粉颗粒中水质子的限制更大。除了PS的溶胀能力和碳水化合物的浸出能力降低外,与PS的阳离子相互作用阻碍了其与水形成氢键的能力,从而限制了溶胀。在WS中,阳离子只能与淀粉的羟基相互作用,对质子迁移、膨胀和糊化的影响较弱。这些发现表明,PS中的磷酸基团通过改变其与水的分子水平相互作用而显著影响其功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


