Comparative studies on the lithium and sodium ions storages in semi-coke derived carbon anode materials
IF 9.2
2区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
Abstract
The same carbon material usually shows different electrochemical performances as the anodes in lithium-ion and sodium-ion batteries, and the underneath mechanisms are remained to be debated. In the work, we prepared more than ten kinds of carbon materials with semi-coke as precursor through calcination in Ar atmosphere at different temperatures from 600 to 2800 °C. The carbon materials prepared at the temperatures from 600 to 1000 °C showed manifest specific capacities for Li and Na ion storage. The ones got at the temperatures from 1200 to 1400 °C delivered a higher Na ion storage capacity than that of Li ion. Oppositely, the carbon materials obtained at the temperatures from 1600 to 2800 °C exhibit higher Li ion storage capacity than that of the Na ion. The structure evolution demonstrates that low-temperature carbon (600–1000 °C) assume excellent long cycling stability for both LIBs and SIBs, but they exhibited lower initial coulombic efficiency (ICE) due to the residual more oxygen-containing groups. The medium-temperature carbon (1200–1400 °C) assume certain nano-sized closed pores that are essential for Na ion storage as a quasi-metallic state, but not the Li ion. This nano-sized closed pores structural features enhance the reversible capacity of medium-temperature carbon but negatively impact their kinetic properties. In contrast, the high-temperature carbon (1600–2800 °C) show graphite gallery that affords with a long cycling performance and high ICE for LIBs, but not for SIBs. This work provides a protocol for synthesizing various carbon materials from semi-coke, offers insights into the electrochemical mechanisms of lithium and sodium storage, and establishes a framework for designing suitable anode materials for LIBs and SIBs.
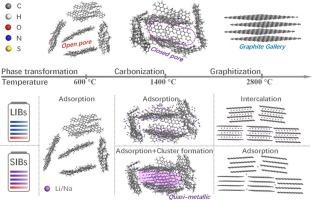
半焦衍生碳负极材料中锂、钠离子储存的比较研究
在锂离子电池和钠离子电池中,相同的碳材料通常表现出不同的电化学性能,其背后的机制仍然存在争议。在600 ~ 2800℃的不同温度下,以半焦为前驱体,在Ar气氛中煅烧制备了十余种碳材料。在600 ~ 1000℃的温度下制备的碳材料显示出Li和Na离子的特异存储能力。在1200 ~ 1400℃的温度下得到的Na离子的存储容量比Li离子的存储容量高。相反,在1600 ~ 2800℃温度下得到的碳材料表现出比Na离子更高的Li离子存储能力。结构演化表明,低温碳(600 ~ 1000℃)对lib和SIBs都具有良好的长循环稳定性,但由于残余的含氧基团较多,它们的初始库仑效率(ICE)较低。中温碳(1200-1400°C)具有一定的纳米尺寸的封闭孔,这些孔对于Na离子作为准金属状态的存储是必不可少的,但对于Li离子则不是。这种纳米尺度的闭孔结构增强了中温碳的可逆性能,但对其动力学性能产生了负面影响。相比之下,高温碳(1600-2800°C)的石墨画廊为lib提供了长循环性能和高ICE,但对于sib则没有。本研究为从半焦中合成各种碳材料提供了一种方案,为锂和钠的电化学储存机制提供了见解,并为设计适合锂离子电池和锂离子电池的阳极材料建立了框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sustainable Materials and Technologies
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
13.40
自引率
4.20%
发文量
158
审稿时长
45 days
期刊介绍:
Sustainable Materials and Technologies (SM&T), an international, cross-disciplinary, fully open access journal published by Elsevier, focuses on original full-length research articles and reviews. It covers applied or fundamental science of nano-, micro-, meso-, and macro-scale aspects of materials and technologies for sustainable development. SM&T gives special attention to contributions that bridge the knowledge gap between materials and system designs.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


