Thiophosphoryltriamide encapsulated into magnetic MCM-41-NH2 as a novel magnetically recoverable mesoporous adsorbent for Hg2+ removal in wastewater: Crystal structure and molecular calculations
IF 6.7
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
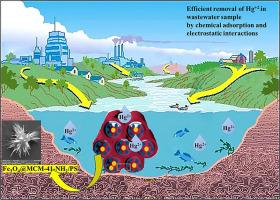
Water contaminants using heavy metal ions particularly Hg2+, due to harmful effects on human health and aquatic ecosystem, are challenging global environmental impact. Hence, the objective of this work is the synthesis of a new absorbent thiophosphoric triamide (PS) decorated on magnetic amine-functionalized mesoporous silica (MCM-41-NH2) for Hg2+ removal. First, HgCl2[P(S)(C6H11NH)3]2 (C) was synthesized by reaction of mercuric chloride and the thiophosphoric triamide ligand (PS). A distorted tetrahedral geometry surrounding the Hg2+ ion was revealed for the obtained complex. Nano-cubic structures of complex and its corresponding ligand (PS´ and Ć) were prepared with size between 60 and 80 nm at chloroform solvent as well. According to the strong interaction of PS to coordination with mercury (II), a newly thiophosphoryl functionalized magnetic mesoporous silica adsorbent, named Fe3O4@MCM-41-NH2/PS, was synthesized with narrow pore size distribution, high specific surface area, and total pore volume, respectively, 7.726 nm, 93.353 m2/g, and 0.137 cm3/g. Initial concentration of Hg2+, adsorbent dosage, pH, temperature, and interfering ions were studied to eliminate Hg2+ from aqueous solution. Moreover, the material exhibited excellent recyclability owing to its magnetic properties, facilitating easy separation and reuse. The adsorption behavior of Hg2+ onto Fe3O4@MCM-41-NH2/PS was best described by the Langmuir isotherm and pseudo-second-order kinetic models and the maximum adsorption capacity was determined to be 161.29 mg g−1 at pH 8 and the temperature of 25 °C. Further, Monte Carlo simulations exhibited the decisive role of S, O, and NH groups for the elevated adsorption of mercury ions on Fe3O4@MCM-41-NH2/PS in the presence of water.
磁性MCM-41-NH2包封的新型磁可回收介孔吸附剂硫代磷酰三酰胺:晶体结构和分子计算
使用重金属离子特别是Hg2+的水污染物,由于对人类健康和水生生态系统的有害影响,正在挑战全球环境影响。因此,本研究的目的是合成一种以磁性胺功能化介孔二氧化硅(MCM-41-NH2)修饰的新型吸附剂硫磷三酰胺(PS),以去除Hg2+。首先,用氯化汞与硫磷三酰胺配体(PS)反应合成HgCl2[P(S)(c6h11nh3)]2 (C)。所得到的配合物呈现出围绕Hg2+离子的扭曲四面体几何形状。在氯仿溶剂下制备了尺寸为60 ~ 80 nm的配合物及其配体(PS´和Ć)的纳米立方结构。根据PS与汞(II)的强配位相互作用,合成了一种新型硫磷基功能化磁性介孔二氧化硅吸附剂Fe3O4@MCM-41-NH2/PS,其孔径分布窄,比表面积大,总孔体积分别为7.726 nm, 93.353 m2/g, 0.137 cm3/g。研究了Hg2+的初始浓度、吸附剂用量、pH、温度和干扰离子对水溶液中Hg2+的去除效果。此外,由于其磁性,该材料具有良好的可回收性,易于分离和再利用。Langmuir等温线和拟二级动力学模型可以很好地描述Hg2+在Fe3O4@MCM-41-NH2/PS上的吸附行为,在pH 8和温度25 °C下,最大吸附量为161.29 mg g−1。此外,蒙特卡罗模拟显示了S、O和NH基团在存在水的情况下对Fe3O4@MCM-41-NH2/PS上汞离子的高吸附起决定性作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


