l-Proline driven Knoevenagel condensation for Assembling benzimidazole-pyrazole-coumarin frameworks; Mechanistic, biological and electrochemical lead sensor applications
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Novel conjugates of substituted pyrazoles linked to 4-hydroxy coumarins and benzimidazoles were synthesized through a l-proline catalyzed multicomponent ligation. The formulated compounds (4a-h) were evaluated in-vitro for antibacterial (broth dilution), anti-tubercular assay (MABA). Some of the screened entities rendered promising in-vitro antibacterial activity (4f-MIC:125 μg/mL), anti-tubercular activity (4b, 4f, 4g-MIC:6.25 μg/mL). Molecular docking examination was executed onto GlmU (N-acetylglucosamine-1-phosphate uridyltransferase, compound 4f showcased strong active site interactions and minimal binding energy. The DFT studies offered valuable insights into chemical reactivity descriptors. The CPE/BPCH modified electrode demonstrated outstanding electrochemical performance for lead detection, offering high sensitivity, low LOD, and excellent stability.
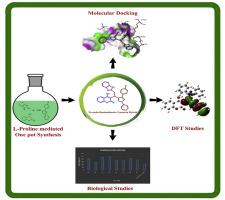
l-脯氨酸驱动Knoevenagel缩合组装苯并咪唑-吡唑-香豆素框架机械,生物和电化学引线传感器的应用
通过l-脯氨酸催化的多组分连接合成了取代吡唑与4-羟基香豆素和苯并咪唑的新型偶联物。对配制的化合物(4a-h)进行体外抗菌(肉汤稀释)、抗结核试验(MABA)评价。筛选的部分实体具有良好的体外抗菌活性(4f- mic:125 μg/mL),抗结核活性(4b, 4f, 4g-MIC:6.25 μg/mL)。对GlmU (n -乙酰氨基葡萄糖-1-磷酸尿苷基转移酶)进行分子对接检测,化合物4f表现出较强的活性位点相互作用和最小的结合能。DFT研究为化学反应性描述符提供了有价值的见解。CPE/BPCH修饰电极在铅检测方面表现出优异的电化学性能,具有高灵敏度、低LOD和优异的稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


