Ferrioxamine X1 mediated iron interaction with FoxA receptor in marine Pseudomonas stutzeri: An in silico approach
IF 2.5
3区 地球科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
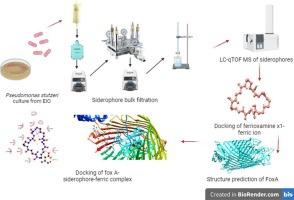
The low iron concentrations in the Equatorial Indian Ocean (EIO) create a challenging environment for microbial life, requiring microorganisms to adapt. One strategy involves the production of siderophores, organic compounds that bind to Fe3+ ions and facilitate their uptake through specific receptors. Multiple siderophores may improve the bioavailability of iron due to the varied structures of siderophores and receptors. Since a single receptor may bind multiple siderophores, it is imperative to understand the molecular mechanisms underlying the interaction between siderophores and their receptors. This study used an in silico approach to explore these interactions. Pseudomonas stutzeri ATCC 17588, isolated from the EIO, was optimized for siderophore production under various growth conditions and analyzed using LC-qTOF-MS. The tertiary structure of the FoxA receptor and its interaction with the ferrioxamine X1-Fe3+ complex were examined through molecular docking. P. stutzeri ATCC 17588 produced multiple siderophores, including ferrioxamine X1, with optimal production at 10 nM iron concentration, pH 8.5, and 25 °C. The interaction energies between ferrioxamine X1-Fe3+ and the FoxA receptor were − 40.81 kcal/mol and − 9.3 kJ/mol, respectively, suggesting stable complexes. The predicted FoxA structure, validated through various analyses, revealed helices interspersed with strands. Hydrophobic interactions, involving residues such as Gln287, Arg792, and Glu180, were primarily responsible for the binding of ferrioxamine X1-Fe3+ to the FoxA receptor. This study sheds light on the role of ferrioxamine X1 in iron acquisition by P. stutzeri in the iron-limited EIO and enhances our understanding of microbial metal-ligand interactions in marine ecosystems.
铁胺X1介导的铁与FoxA受体在海洋stutzeri假单胞菌中的相互作用:一种计算机方法
赤道印度洋(EIO)的低铁浓度为微生物生命创造了一个具有挑战性的环境,需要微生物适应。一种策略涉及到铁载体的产生,这是一种与铁离子结合并促进其通过特定受体吸收的有机化合物。由于铁载体和受体的结构不同,多种铁载体可以提高铁的生物利用度。由于单一受体可以结合多个铁载体,因此了解铁载体与其受体之间相互作用的分子机制是必要的。本研究使用了一种计算机方法来探索这些相互作用。从EIO中分离得到假单胞菌stutzeri ATCC 17588,对其在不同生长条件下产铁载体进行了优化,并用LC-qTOF-MS对其进行了分析。通过分子对接研究了FoxA受体的三级结构及其与铁胺X1-Fe3+络合物的相互作用。P. stutzeri ATCC 17588产生多种铁载体,包括铁胺X1,铁浓度为10 nM, pH为8.5,温度为25°C。铁胺X1-Fe3+与FoxA受体的相互作用能分别为- 40.81 kcal/mol和- 9.3 kJ/mol,配合物稳定。预测的FoxA结构,通过各种分析验证,显示出螺旋穿插着链。涉及Gln287、Arg792和Glu180等残基的疏水相互作用是铁胺X1-Fe3+与FoxA受体结合的主要原因。该研究揭示了铁胺X1在P. stutzeri在铁受限的EIO中获取铁的作用,并增强了我们对海洋生态系统中微生物金属-配体相互作用的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Marine Chemistry
化学-海洋学
CiteScore
6.00
自引率
3.30%
发文量
70
审稿时长
4.5 months
期刊介绍:
Marine Chemistry is an international medium for the publication of original studies and occasional reviews in the field of chemistry in the marine environment, with emphasis on the dynamic approach. The journal endeavours to cover all aspects, from chemical processes to theoretical and experimental work, and, by providing a central channel of communication, to speed the flow of information in this relatively new and rapidly expanding discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


