Fabrication of green ternary CeO2@Co3O4@g-C3N4 nanocomposite as an efficient and durable sorbent for the ciprofloxacin removal
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The issue of water resource pollution resulting from the discharge of pharmaceutical residues has become of great concern for the environment. In this investigation, we describe the first-ever production of a green CeO2@Co3O4@C3N4 (CeCoCN) as an exuberant potential nanosorbent for the ciprofloxacin (CIP) removal from aqueous system. Analytical tools such as XPS, XRD, FTIR, BET, FTIR, EDX, SEM, and TEM were used to characterize the produced adsorbent. According to the Langmuir model, the CeCoCN nanosorbent has an adsorption capability of 325 mg/g. The rate of removal is controlled by both the number of adsorption sites and the concentration of CIP in water. This was shown by the adsorptive uptake kinetics, which followed pseudo-second-order (PSO) kinetics. With an adsorption efficiency of 86.7 % and good structural stability data from XRD and FTIR, the CeCoCN nanosorbent was reusable for four cycles. Hydrogen bonding, electrostatic attraction, pore diffusion, complexation, and the π-π interaction were the primary mechanisms of uptake. Our research indicates that the adsorbents are a stable, effective, and recyclable nanosorbent for CIP adsorption.
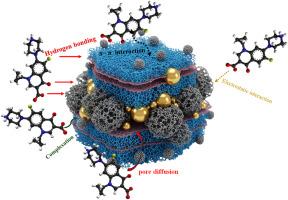
绿色三元化合物CeO2@Co3O4@g-C3N4纳米复合材料的制备及其对环丙沙星的吸附效果
药物残留排放造成的水资源污染问题已成为人们关注的环境问题。在这项研究中,我们描述了绿色CeO2@Co3O4@C3N4 (CeCoCN)的首次生产,作为一种具有旺盛潜力的纳米吸附剂,用于从水系统中去除环丙沙星(CIP)。采用XPS、XRD、FTIR、BET、FTIR、EDX、SEM、TEM等分析工具对制备的吸附剂进行了表征。根据Langmuir模型,CeCoCN纳米吸附剂的吸附能力为325 mg/g。去除速率受吸附位点数目和水中CIP浓度的共同控制。这是由吸附摄取动力学表明,它遵循伪二阶(PSO)动力学。通过XRD和FTIR测试,ceocn纳米吸附剂的吸附效率为86.7%,结构稳定性良好,可重复使用4次。氢键、静电吸引、孔隙扩散、络合和π-π相互作用是吸附的主要机制。研究表明,该吸附剂是一种稳定、有效、可循环利用的CIP吸附纳米吸附剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


