Comprehensive adsorption study of methyl red onto MFe2O4 using DFT calculations and Monte Carlo simulations
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
This work aims to elucidate the chemical properties of methyl red (MR) and the mechanisms underlying its interactions with MFe2O4 (311) (M = Co, Cu, Mg, and Zn) in aqueous environment. Quantum computational descriptors were calculated based on the lowest unoccupied molecular orbital energy and the highest occupied molecular orbital energy. Additionally, Molecular descriptors were obtained through the Adsorption Locator module. The minimal ΔEgap indicates a high reactivity of MR, which correlates with adsorption configurations predominantly adopting a parallel orientation relative to the surfaces. The dynamic descriptors reveal that the adsorption mechanism is spontaneous and exothermic. Accordingly, the MFe2O4/MR/500H2O systems exhibited higher adsorption energies, ranging from −776.08 to −703.98 kcal/mol, compared to MFe2O4/MR/H2O systems with fewer water molecules (400, 300, and 100H2O). Among the MFe2O4/MR/500H2O systems, the MR/CuFe2O4 complex demonstrated the highest Eads at −776.08 kcal/mol, followed by MR/CoFe2O4 (−723.74 kcal/mol), MR/MgFe2O4 (−713.35 kcal/mol), and MR/ZnFe2O4 (−703.98 kcal/mol). Across all studied systems, dEads/dNiMR values significantly exceed the dEads/dNiH2O values, implying that H2O molecules on the MFe2O4 surface could be partially substituted. The most stable configurations imply chemisorption between the adsorbate and the surfaces, a conclusion further supported by radial distribution function (RDF) analysis.
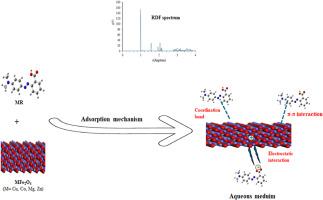
用DFT计算和蒙特卡罗模拟研究甲基红在MFe2O4上的综合吸附
本工作旨在阐明甲基红(MR)的化学性质及其在水环境中与MFe2O4 (311) (M = Co, Cu, Mg和Zn)相互作用的机制。量子计算描述子是基于最低的未占据分子轨道能量和最高的已占据分子轨道能量来计算的。此外,通过吸附定位器模块获得分子描述符。最小的ΔEgap表示MR的高反应性,这与吸附构型主要采用相对于表面的平行取向有关。动力学描述符表明,吸附机制是自发的、放热的。因此,与水分子较少的MFe2O4/MR/H2O体系(400、300和100H2O)相比,MFe2O4/MR/500H2O体系表现出更高的吸附能,在−776.08 ~−703.98 kcal/mol之间。在MFe2O4/MR/500H2O体系中,MR/CuFe2O4配合物的Eads最高,为- 776.08 kcal/mol,其次是MR/CoFe2O4 (- 723.74 kcal/mol)、MR/MgFe2O4 (- 713.35 kcal/mol)和MR/ZnFe2O4 (- 703.98 kcal/mol)。在所有研究体系中,dEads/ dniimr值显著超过dEads/dNiH2O值,这意味着MFe2O4表面的H2O分子可以被部分取代。最稳定的构型意味着吸附质与表面之间的化学吸附,这一结论进一步得到了径向分布函数(RDF)分析的支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


