Active site on boron‑nitrogen co-doping lignin-based carbon nanotube-coated nZVI for enhanced hexavalent chromium removal by adsorption-redox behavior from groundwater
IF 6.7
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The utilization of high-efficiency and environmentally friendly materials for pollutant removal has remained a research hotspot in groundwater remediation studies. Herein, a boron (B) and nitrogen (N) co-doped lignin-based carbon nanotube-coated nano zero-valent iron (nZVI) composite (Fe0@LC-NB) was synthesized via a straightforward one-step pyrolysis protocol for Cr(VI) remediation from groundwater. This synthetic approach simultaneously achieves the carbonization of lignin, growth of carbon nanotubes (CNTs), formation of nZVI, and co-doping of boron and nitrogen, resulting in a tubular structure that effectively prevents the aggregation of nZVI and enhances its reactivity. The abundant active sites of B–N–C, B![]() C, and pyridinic N generated by B/N co-doping are absent in singly-doped materials, significantly boosting the Cr(VI) adsorption capacity. Simultaneously, the electron donor-acceptor system formed by B, N, and Fe0 markedly increases the interfacial electron transfer rate, thereby achieving exceptional redox activity of the material. The results showed that the maximal adsorption capacity of Fe0@LC-NB reached 232.94 mg/g, which was 1.5 times that of un-modified lignin-based carbon nanotube-coated nZVI (Fe0@LC). The mechanism analysis indicates that electrostatic adsorption, redox reactions, and complexation processes were the major reasons for Cr(VI) removal. Moreover, unlike conventional nZVI materials that undergo rapid passivation in application, the protective carbon shell enables the composite to maintain outstanding performance even in complex aquatic environments, offering a cost-effective and efficient method for groundwater remediation.
C, and pyridinic N generated by B/N co-doping are absent in singly-doped materials, significantly boosting the Cr(VI) adsorption capacity. Simultaneously, the electron donor-acceptor system formed by B, N, and Fe0 markedly increases the interfacial electron transfer rate, thereby achieving exceptional redox activity of the material. The results showed that the maximal adsorption capacity of Fe0@LC-NB reached 232.94 mg/g, which was 1.5 times that of un-modified lignin-based carbon nanotube-coated nZVI (Fe0@LC). The mechanism analysis indicates that electrostatic adsorption, redox reactions, and complexation processes were the major reasons for Cr(VI) removal. Moreover, unlike conventional nZVI materials that undergo rapid passivation in application, the protective carbon shell enables the composite to maintain outstanding performance even in complex aquatic environments, offering a cost-effective and efficient method for groundwater remediation.
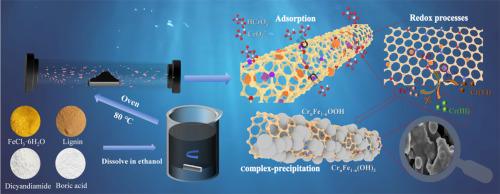
硼氮共掺杂木质素基碳纳米管包覆nZVI对地下水中六价铬的吸附氧化还原去除活性位点
利用高效环保的材料去除污染物一直是地下水修复研究的热点。本文采用一步热解法合成了硼(B)和氮(N)共掺杂木质素基碳纳米管包覆纳米零价铁(nZVI)复合材料(Fe0@LC-NB),用于地下水中Cr(VI)的修复。该合成方法同时实现了木质素的炭化、碳纳米管的生长、nZVI的形成以及硼氮的共掺杂,形成管状结构,有效地阻止了nZVI的聚集,提高了其反应活性。B/N共掺杂产生的丰富的B - N - c、BC和吡啶N活性位点在单掺杂材料中不存在,显著提高了Cr(VI)的吸附能力。同时,由B、N和Fe0组成的电子供体-受体体系显著提高了界面电子传递速率,从而实现了材料优异的氧化还原活性。结果表明,Fe0@LC-NB的最大吸附量为232.94 mg/g,是未改性木质素基碳纳米管包覆nZVI (Fe0@LC)的1.5倍。机理分析表明,静电吸附、氧化还原反应和络合反应是去除Cr(VI)的主要原因。此外,与传统的nZVI材料在应用过程中经历快速钝化不同,保护性碳壳使复合材料即使在复杂的水生环境中也能保持出色的性能,为地下水修复提供了一种经济高效的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


