Efficient trans-aconitic acid production using systematically metabolic engineered Escherichia coli
IF 4.4
2区 生物学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
trans-Aconitic acid (TAA) is a versatile platform biochemical exhibiting extensive applications. In this work, Escherichia coli W3110 (DE3) was metabolic modified for the de novo biosynthesis of TAA. Firstly, a pyruvate accumulation chassis strain E. coli W3110-P6 was constructed through deletion of six byproducts generation genes. Secondly, the aconitate isomerase was screened from four candidates and co-overexpressed with TAA transporter to construct heterologous TAA biosynthetic pathway in E. coli W3110-P6. Thirdly, the genes pycP458S encoding a feedback-insensitive pyruvate carboxylase and gltA encoding an allosterically unaffected citrate synthase were overexpressed, and aceA encoding the isocitrate lyase was deleted, to increase precursor supply and TAA generation of the recombinant E. coli. Finally, the fermentation condition of the obtained strain E. coli W3110-TAA08 was optimized. TAA at a concentration of 37.32 g/L was generated within 40 h, with a yield of 0.76 g/g glucose and a productivity of 0.93 g/L/h.
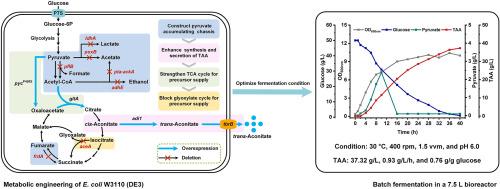
利用系统代谢工程大肠杆菌高效生产反式乌头酸
反式乌头酸(TAA)是一种用途广泛的生物化学平台。本研究对大肠杆菌W3110 (DE3)进行了代谢修饰,用于TAA的从头生物合成。首先,通过删除6个副产物产生基因,构建丙酮酸积累底盘菌株W3110-P6。其次,从4个候选物中筛选出乌头酸异构酶,与TAA转运蛋白共过表达,在大肠杆菌W3110-P6中构建异源TAA生物合成途径。再次,将编码反馈不敏感型丙酮酸羧化酶的基因pycP458S和编码变构不影响型柠檬酸合成酶的基因gltA过表达,删除编码异柠檬酸裂解酶的基因aceA,以增加重组大肠杆菌前体供应和TAA的生成。最后,对所得菌株W3110-TAA08的发酵条件进行了优化。在40 h内生成浓度为37.32 g/L的TAA,产率为0.76 g/g葡萄糖,产率为0.93 g/L/h。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synthetic and Systems Biotechnology
BIOTECHNOLOGY & APPLIED MICROBIOLOGY-
CiteScore
6.90
自引率
12.50%
发文量
90
审稿时长
67 days
期刊介绍:
Synthetic and Systems Biotechnology aims to promote the communication of original research in synthetic and systems biology, with strong emphasis on applications towards biotechnology. This journal is a quarterly peer-reviewed journal led by Editor-in-Chief Lixin Zhang. The journal publishes high-quality research; focusing on integrative approaches to enable the understanding and design of biological systems, and research to develop the application of systems and synthetic biology to natural systems. This journal will publish Articles, Short notes, Methods, Mini Reviews, Commentary and Conference reviews.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


