Elevated FBXO45 promotes TFG ubiquitination and drives lung metastasis of hepatocellular carcinoma
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
Elevated F-box/SPRY domain protein 1 (FBXO45) expression is associated with human hepatocellular carcinoma (HCC). While TP53 mutations represent a common genetic alteration in HCC, the potential regulatory interplay between TP53 and FBXO45 in modulating HCC progression remains unclear.
Methods
We analyzed the relationship between TP53 and FBXO45 in patients with HCC. A series of assays were also performed to explore the effect of FBXO45 on invasion and metastasis in TP53-mutated HCC cell lines and mouse models. These experimental findings were further validated in clinical samples.
Results
FBXO45 expression was upregulated in 78.3% (83/106) of patients with HCC with TP53 mutation, marking an aggressive subtype. Overexpression of FBXO45 significantly enhanced HCC cell migration and invasion capacity (p <0.001), whereas FBXO45 silencing suppressed these effects (p <0.01). Mechanistically, FBXO45 promoted Trk-fused gene (TFG) Lys103 ubiquitination and then increased its stability. This post-translational modification facilitates the binding of transcription factor activating transcription factor 2 (ATF2), leading to subsequent upregulation of nuclear factor-kappa B (NF-κB) p65 expression and ultimately promoting the migratory and invasive properties of TP53-mutant HCC cells. In vivo validation using an orthotopic xenograft model showed that FBXO45 overexpression significantly promoted HCC lung metastasis incidence (7/9 mice with metastases), whereas TFG knockdown abrogated this metastatic potential. Clinically, patients exhibiting high co-expression of FBXO45 and TFG defined an aggressive HCC subtype with significantly worse prognosis (p = 0.0015).
Conclusions
Our results provide new mechanistic insight into the role of FBXO45 in driving HCC invasion and metastasis. Therefore, targeting the TP53–FBXO45–TFG–ATF2–NF-κB axis represents a promising therapeutic approach for treating aggressive HCC with TP53 mutations.
Impact and implications
This study highlights that FBXO45 is highly expressed in patients with HCC with TP53 mutations. FBXO45 promotes HCC lung metastasis by activating the TFG–ATF2–NF-κB–epithelial–mesenchymal transition signaling axis. In addition, we found that targeting the TP53–FBXO45–TFG–ATF2–NF-κB axis could be a novel approach for the treatment of metastatic HCC.
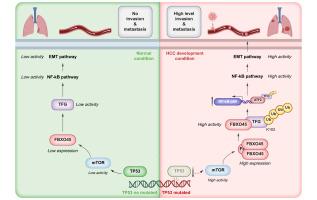
FBXO45升高可促进TFG泛素化,促进肝细胞癌肺转移
F-box/SPRY结构域蛋白1 (FBXO45)表达升高与人肝细胞癌(HCC)有关。虽然TP53突变代表HCC中常见的遗传改变,但TP53和FBXO45在调节HCC进展中的潜在调节相互作用仍不清楚。方法分析肝癌患者TP53与FBXO45的关系。在tp53突变的肝癌细胞系和小鼠模型中,通过一系列实验探讨FBXO45对肝癌侵袭转移的影响。这些实验结果在临床样品中得到了进一步的验证。结果78.3%(83/106)的肝癌TP53突变患者fbxo45表达上调,为侵袭性亚型。过表达FBXO45可显著增强HCC细胞的迁移和侵袭能力(p <0.001),而沉默FBXO45可抑制这些作用(p <0.01)。机制上,FBXO45促进trk融合基因(TFG) Lys103泛素化,进而提高其稳定性。这种翻译后修饰促进了转录因子活化转录因子2 (ATF2)的结合,导致随后核因子κB (NF-κB) p65的表达上调,最终促进了tp53突变型HCC细胞的迁移和侵袭特性。原位异种移植模型的体内验证表明,FBXO45过表达显著提高了HCC肺转移发生率(7/9的转移小鼠),而TFG敲低则消除了这种转移潜力。临床上,FBXO45和TFG高表达的患者定义为侵袭性HCC亚型,预后明显较差(p = 0.0015)。结论我们的研究结果为FBXO45在HCC侵袭转移中的作用提供了新的机制。因此,靶向TP53 - fbxo45 - tfg - atf2 - nf -κB轴是治疗TP53突变侵袭性HCC的一种有希望的治疗方法。影响和意义本研究强调FBXO45在TP53突变的HCC患者中高表达。FBXO45通过激活TFG-ATF2-NF -κ b -上皮-间质转化信号轴促进HCC肺转移。此外,我们发现靶向TP53-FBXO45-TFG-ATF2-NF -κB轴可能是治疗转移性HCC的新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


