Copper-based electrochemical sensor derived from halogen-substituted Schiff base for selective detection of neurotransmitter dopamine: Insight from DFT and docking analysis
IF 4.2
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
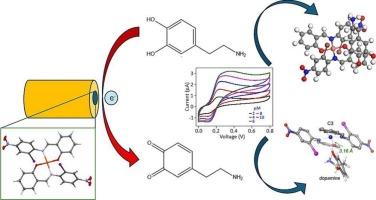
The significant influence of dopamine levels on biological processes and diseases necessitates precise and selective detection methods, crucial for conditions like Parkinson's and Alzheimer's diseases. In this study, copper(II) complexes (C1-C3) derived from halogen-substituted Schiff base ligands were synthesized in situ without isolating the ligands, employing methanol as a solvent at room temperature. Characterization through FTIR, UV–Vis, elemental analysis, and mass spectroscopy, along with single crystal X-ray diffraction (SCXRD) analysis, unveiled the solid-state structures of the complexes. Notably, the ligands acted as bidentate mono-negative, coordinating the Cu(II) ion through oxygen and nitrogen atoms, resulting in a square planar geometry. These modified complexes were applied to glassy carbon electrodes (GCEs) for electrochemical sensing of dopamine using differential pulse voltammetry (DPV) and cyclic voltammetry (CV) methods, with dopamine concentrations ranging from 2 to 10 μM. While C2 did not detect dopamine at any tested concentrations, C1 and C3 emerged as effective electrode materials for dopamine sensing, with C3 exhibiting superior performance by providing an effective surface area for the electrochemical oxidation of dopamine. The sensitivity and limits of detection (LOD) for C1 and C3 were determined to be 0.72 μAcm−2 μM−1 and 1.67 μM, and 3.72 μAcm−2 μM−1 and 0.52 μM, respectively. The proposed method exhibited no interference by γ-amino butyric acid, uric acid, ascorbic acid, and glucose at concentrations of 15 μM. Additionally, results from computational studies involving DFT, molecular docking, and molecular dynamic simulations not only supported the experimental findings but also elucidated the interaction mechanisms between the compounds and dopamine. These findings position the electrochemical sensors, particularly C3, as promising candidates for the development of sensitive and low-limit electrochemical sensors.
基于卤素取代希夫碱的铜基电化学传感器用于神经递质多巴胺的选择性检测:来自DFT和对接分析的见解
多巴胺水平对生物过程和疾病的重大影响需要精确和选择性的检测方法,这对帕金森病和阿尔茨海默病等疾病至关重要。在本研究中,以甲醇为溶剂,在室温下原位合成了由卤素取代的席夫碱配体衍生的铜(II)配合物(C1-C3)。通过FTIR, UV-Vis,元素分析,质谱以及单晶x射线衍射(SCXRD)分析,揭示了配合物的固态结构。值得注意的是,配体是双齿单负的,通过氧和氮原子配位Cu(II)离子,形成方形平面几何形状。在多巴胺浓度范围为2 ~ 10 μM的条件下,利用差分脉冲伏安法(DPV)和循环伏安法(CV)将这些修饰的配合物应用于玻璃碳电极(GCEs)上进行多巴胺的电化学传感。C2在任何测试浓度下都不能检测到多巴胺,而C1和C3成为多巴胺传感的有效电极材料,其中C3通过为多巴胺的电化学氧化提供有效的表面积而表现出优越的性能。C1和C3的灵敏度和检出限分别为0.72 μAcm−2 μM−1和1.67 μM, 3.72 μAcm−2 μM−1和0.52 μM。在15 μM的浓度下,γ-氨基丁酸、尿酸、抗坏血酸和葡萄糖对该方法无干扰。此外,包括DFT、分子对接和分子动力学模拟在内的计算研究结果不仅支持了实验结果,而且阐明了化合物与多巴胺之间的相互作用机制。这些发现使电化学传感器,特别是C3,成为开发灵敏和低极限电化学传感器的有希望的候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


