Manipulating the oxidation state of Cu ions in BaAlBO3F2 via X-rays and thermal treatment
IF 5.7
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The oxidation state of Cu in BaAlBO₃F₂ (BABF) is reduced to Cu+ by ionizing radiation. In the as-prepared state, the higher oxidation state of Cu is considered to occupy two different crystallographic sites (A and B), which are possibly the five-coordinate Al3+ and the twelve-coordinate Ba2+ site, respectively. Upon X-ray ionization of the host matrix, electrons are selectively captured by the Cu ion at the B site, reducing the oxidation state to the monovalent Cu+ state. This reduction leads to a photoluminescence (PL) band peaking at 520 nm under UV excitation, corresponding to the 3d94s1 → 3d10 transition of Cu+. The evolution of the PL band (or the formation of a luminescent center) induced by ionizing radiation is referred to as radiophotoluminescence (RPL). The RPL emission intensity is proportional to the X-ray irradiation dose and can be reversed by heat treatment. During heat treatment at temperatures ranging from 100 to 200 °C, electrons from the Cu+ center at the B site are transferred to the higher oxidation state of Cu at the A site. Consequently, another Cu+ center is formed at the A site, resulting in a PL band peaking at 580 nm, while the intensity of the 520 nm peak decreases due to the depopulation of Cu+ at the B site. Furthermore, increasing the treatment temperature above 350 °C reverses the electron transfer at the A site. The formation and annihilation of Cu centers with different oxidation states are reproducible multiple times.
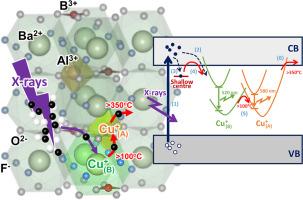
通过x射线和热处理控制BaAlBO3F2中Cu离子的氧化态
BaAlBO₃F₂(BABF)中Cu的氧化态通过电离辐射还原为Cu+。在制备状态下,Cu的高氧化态被认为占据了两个不同的晶体位置(A和B),可能分别是五坐标Al3+和十二坐标Ba2+。在x射线电离基体后,电子在B位被Cu离子选择性捕获,将氧化态还原为单价Cu+态。这种还原导致在UV激发下,光致发光(PL)带在520 nm处达到峰值,对应于Cu+的3d94s1→3d10跃迁。由电离辐射引起的PL波段的演变(或发光中心的形成)被称为放射光致发光(RPL)。RPL发射强度与x射线辐照剂量成正比,可通过热处理逆转。在100 ~ 200℃的热处理过程中,B位Cu+中心的电子转移到A位更高氧化态的Cu上。因此,在A位点形成另一个Cu+中心,导致在580nm处出现一个PL峰,而由于B位点Cu+的减少,520nm峰的强度降低。此外,将处理温度提高到350℃以上会逆转A位的电子转移。不同氧化态Cu中心的形成和湮灭是可重复多次的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Research Bulletin
工程技术-材料科学:综合
CiteScore
9.80
自引率
5.60%
发文量
372
审稿时长
42 days
期刊介绍:
Materials Research Bulletin is an international journal reporting high-impact research on processing-structure-property relationships in functional materials and nanomaterials with interesting electronic, magnetic, optical, thermal, mechanical or catalytic properties. Papers purely on thermodynamics or theoretical calculations (e.g., density functional theory) do not fall within the scope of the journal unless they also demonstrate a clear link to physical properties. Topics covered include functional materials (e.g., dielectrics, pyroelectrics, piezoelectrics, ferroelectrics, relaxors, thermoelectrics, etc.); electrochemistry and solid-state ionics (e.g., photovoltaics, batteries, sensors, and fuel cells); nanomaterials, graphene, and nanocomposites; luminescence and photocatalysis; crystal-structure and defect-structure analysis; novel electronics; non-crystalline solids; flexible electronics; protein-material interactions; and polymeric ion-exchange membranes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


