Optimization of oxygen evolution performance with multi-component high entropy alloy catalyst
IF 5.7
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
High-entropy alloys (HEAs) are excellent catalyst supports due to their large specific surface area, tunable morphology, and uniform distribution of metal ions. With their unique structural characteristics, HEAs can provide more catalytic active sites, thereby improving performance and stability and enhancing synergistic catalytic effects. In this context, researchers have successfully synthesized HEAs-FeNiCoCd catalysts using nickel foam as a substrate via a hydrothermal method. Using nickel foam as a substrate significantly enhances the catalyst's porosity. Observations reveal that the catalyst exhibits a nano-flower-like structure, featuring a large number of active sites and a vast specific surface area, thereby providing more reaction centers and significantly improving catalytic reaction rates and efficiency. The catalyst was tested in a series of experiments using 1 M KOH solution. In the oxygen evolution reaction (OER), at a current density of 10 mA·cm-2, the overpotential was only 150.4 mV, and the Tafel slope was only 58.06 mV·dec-1, with excellent stability. With its outstanding catalytic performance, stability, corrosion resistance, and low cost, the HEAs catalyst will play an important role in more fields.
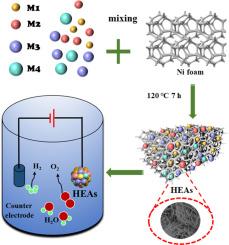
多组分高熵合金催化剂析氧性能优化
高熵合金(HEAs)具有比表面积大、形貌可调、金属离子分布均匀等优点,是优良的催化剂载体。HEAs以其独特的结构特点,可以提供更多的催化活性位点,从而提高性能和稳定性,增强协同催化效果。在此背景下,研究人员通过水热法成功地以泡沫镍为衬底合成了HEAs-FeNiCoCd催化剂。使用泡沫镍作为衬底可以显著提高催化剂的孔隙率。观察结果表明,该催化剂呈纳米花状结构,具有大量的活性位点和广阔的比表面积,从而提供更多的反应中心,显著提高催化反应速率和效率。用1 M KOH溶液对催化剂进行了一系列实验。在析氧反应(OER)中,电流密度为10 mA·cm-2时,过电位仅为150.4 mV, Tafel斜率仅为58.06 mV·dec1,具有良好的稳定性。HEAs催化剂以其优异的催化性能、稳定性、耐腐蚀性和低廉的成本,将在更多的领域发挥重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Research Bulletin
工程技术-材料科学:综合
CiteScore
9.80
自引率
5.60%
发文量
372
审稿时长
42 days
期刊介绍:
Materials Research Bulletin is an international journal reporting high-impact research on processing-structure-property relationships in functional materials and nanomaterials with interesting electronic, magnetic, optical, thermal, mechanical or catalytic properties. Papers purely on thermodynamics or theoretical calculations (e.g., density functional theory) do not fall within the scope of the journal unless they also demonstrate a clear link to physical properties. Topics covered include functional materials (e.g., dielectrics, pyroelectrics, piezoelectrics, ferroelectrics, relaxors, thermoelectrics, etc.); electrochemistry and solid-state ionics (e.g., photovoltaics, batteries, sensors, and fuel cells); nanomaterials, graphene, and nanocomposites; luminescence and photocatalysis; crystal-structure and defect-structure analysis; novel electronics; non-crystalline solids; flexible electronics; protein-material interactions; and polymeric ion-exchange membranes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


