A Cu(I)–Tb(III) heterometallic MOF showing solvatochromic behavior, guest-dependent luminescence and nitrobenzene sensing
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
A heterometallic-organic framework, {[TbCu2I(4-tzba)2(DMF)2(EtOH)2]·2DMF·4H2O}n (TbCu-1), was successfully synthesized under solvothermal conditions using the bifunctional tetrazole-carboxylate ligand, 4-H2tzba (4-H2tzba = 4-(1H-tetrazol-5-yl)benzoic acid). TbCu-1 exhibits a 3D, 4-connected porous framework comprising binuclear [Tb2(COO)4] SBUs and 1D infinite [Cu2I(4-tzba]2] chains. Notably, TbCu-1 shows a visible color change when immersed to certain aromatic molecules (nitrobenzene, aniline, N-methylaniline, N,N-dimethylaniline). Additionally, its weak fluorescence intensifies into strong green Tb3+ emission after the inclusion of special alkyl-benzenes or selected aniline serials (e.g., toluene, xylene, N,N-dimethylaniline, N,N-dimethyl-p-toluidine), whereas nitrobenzene, aniline, and N-methylaniline quench this Tb3+-based fluorescence. However, TbCu-1 only emits ligand-based fluorescence when dispersed in DMF. Its luminescence response to aromatic solvents suggests it can serve as a fast, sensitive fluorescence sensor for nitrobenzene via quenching, with a detection limit of 3.81 × 10−6 M and a KSV value of 9.47 × 103 M−1.
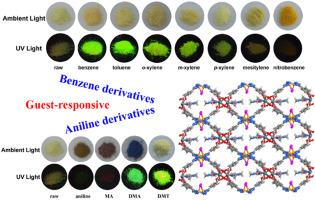
Cu(I) -Tb (III)异质金属MOF具有溶剂致变色行为、客体依赖发光和硝基苯传感
以双功能四唑-羧酸配体4- h2tzba (4- (1h -四唑-5-基)苯甲酸)为配体,在溶剂热条件下成功合成了异金属有机骨架{[TbCu2I(4-tzba)2(DMF)2(EtOH)2]·2DMF·4H2O}n (TbCu-1)。TbCu-1呈现出由双核[Tb2(COO)4] SBUs和一维无限[Cu2I(4-tzba]2]链组成的三维4连接多孔框架。值得注意的是,当浸入某些芳香分子(硝基苯、苯胺、N-甲基苯胺、N,N-二甲基苯胺)时,TbCu-1表现出明显的颜色变化。此外,在加入特殊的烷基苯或选定的苯胺系列(如甲苯、二甲苯、N,N-二甲基苯胺、N,N-二甲基对甲苯胺)后,其弱荧光增强为强绿色Tb3+发射,而硝基苯、苯胺和N-甲基苯胺会猝灭这种Tb3+基荧光。然而,TbCu-1在DMF中分散时仅发出基于配体的荧光。对芳香族溶剂的发光响应表明,通过猝灭可以作为一种快速、灵敏的硝基苯荧光传感器,检测限为3.81 × 10−6 M, KSV值为9.47 × 103 M−1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


