Design and synthesis of aminol- and phenylamide-containing phosphonic acids and their biological activity evaluation
IF 4
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
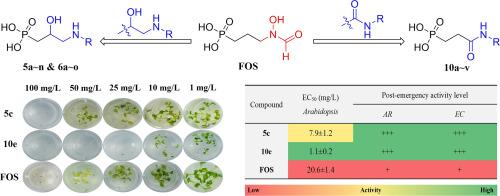
Inhibitor design based on the natural product fosmidomycin (FOS) as a lead structure is a promising strategy in search of herbicidally active compounds that may target the DXR enzyme in the MEP pathway. Herein, two metal ion chelating groups, aminol and amide, were used to replace the hydroxamate moiety of FOS, and two types of non-hydroxamate FOS analogs, namely aminol- (5a ∼ n and 6a ∼ o) and phenylamide-containing (10a ∼ v) phosphonic acids were designed and synthesized by establishing facile routes involving the Michaelis-Arbuzov reaction, epoxide ring-opening, acrylamidation, Michael addition, and phosphonate hydrolysis. In the pre-emergency bioactivity screening several compounds demonstrated herbicidal activities superior to FOS, including 5c, 5 h, 10a, 10d, 10e, etc. In particular, 10e showed an 18.7-fold inhibition activity against Arabidopsis thaliana and 16.1- and 10.8-fold activities against the root and stalk of Echinochloa crus-galli, respectively, in comparison with that of FOS. In the post-emergency assay, 10e also displayed 1.9- and 2.1-fold inhibition activities against E. crus-galli and Amaranthus retroflexus. Enzyme inhibition assay revealed the inhibition activities of some compounds to the DXR enzyme far below that of FOS, while the plant growth rescue failed from the inhibition by 5c and 10e by adding dimethylallyl pyrophosphate (DMAPP), a downstream product of the MEP pathway, both suggesting that some targets instead of the DXR enzyme might be involved in the inhibition by the active compounds. Nevertheless, this work demonstrated that using bioisosteric replacement based on the lead structure FOS is an effective strategy for developing highly herbicidal active compounds.
含氨基和苯胺膦酸的设计合成及其生物活性评价
基于天然产物fosmidomycin (FOS)作为先导结构的抑制剂设计是寻找可能靶向MEP通路中DXR酶的除草活性化合物的一种很有前途的策略。本文采用氨基和酰胺两种金属离子螯合基团取代FOS的羟基部分,通过建立Michaelis-Arbuzov反应、环氧化物开环、丙烯酰胺化、Michael加成和膦酸水解的简便路线,设计并合成了两种非羟基的FOS类似物,即氨基- (5a ~ n和6a ~ o)和含苯胺(10a ~ v)膦酸。在应急前生物活性筛选中,几种化合物表现出优于FOS的除草活性,包括5h、5h、10a、10d、10e等。其中,10e对拟南芥(Arabidopsis thaliana)的抑制活性是FOS的18.7倍,对棘球藻(Echinochloa cross -galli)的根和茎的抑制活性分别是FOS的16.1倍和10.8倍。在应急试验中,10e对大肠杆菌和逆转录苋的抑制活性分别为1.9倍和2.1倍。酶抑制实验显示,部分化合物对DXR酶的抑制活性远低于FOS,而添加MEP途径下游产物二甲基丙烯基焦磷酸(dimethylallyl pyrophosphate, DMAPP)对5c和10e的抑制作用使植物生长恢复失败,这表明活性化合物可能参与了一些靶点而不是DXR酶的抑制作用。然而,本研究表明,利用基于先导结构FOS的生物等构替代是开发高除草活性化合物的有效策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


